Resistin Human ELISA
Resistin, a product of the RSTN gene, is a peptide hormone belonging to the class of cysteinerich secreted proteins which is termed the RELM family, and is also described as ADSF (Adipose Tissue-Specific Secretory Factor) or FIZZ3 (Found in Inflammatory Zone). Human resistin contains 108 amino acids as a prepeptide, and its hydrophobic signal peptide is cleaved before its secretion. Resistin circulates in human blood as a dimeric protein consisting of two 92 amino acid polypeptides, which are disulfide-linked via Cys26.
Much of the early investigations about the resistin molecule are based on the mouse model. Resistin, produced and secreted primarily by adipocytes in mice, acts on skeletal muscle myocytes, hepatocytes and adipocytes themselves so that it reduces their sensitivity to insulin. Steppan et al. have suggested that resistin suppresses the ability of insulin to stimulate glucose uptake. Other studies have shown that mouse resistin increases during the differentiation of adipocytes, but it also seems to inhibit adipogenesis.
Compared to the mouse model, human adipogenic differentiation is likely to be associated with a down regulation of resistin gene expression. On the other hand, resistin was found to be expressed at high levels in human monocytes, macrophages and bone marrow. Recent investigations have shown that human resistin is correlated with metabolic syndrome and obesity-related disorders. Malo et al. have reported that resistin levels are positively associated with waist circumference, tumor necrosis factor-a, and insulin resistance assessed by the homeostasis model, and inversely correlated with total cholesterol, HDL cholesterol, and LDL cholesterol. Moreover, Sadhasiv et al. found a positive correlation of SAT (subcutaneous adipose tissue) resistin mRNA expression with serum resistin, BMI and insulin resistance (HOMA index).
Based on the above reports human resistin might be an important marker that acts as the link between obesity and insulin resistance. Resistin can play a role also in inflammation processes
and in atherosclerosis. Clinical use and areas of investigation: Energy metabolism and body weight regulation, Metabolic syndrome, Inflammation, Atherosclerosis
The RD191016100 Human Resistin ELISA is a sandwich enzyme immunoassay for the quantitative measurement of human resistin in serum and plasma.
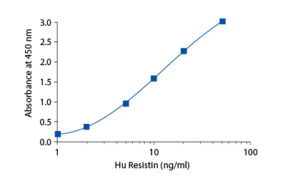
In the BioVendor Human Resistin ELISA, Standards, Quality Controls and samples are incubated in microplate wells pre-coated with polyclonal anti-human resistin antibody. After 60 minutes incubation and washing, biotin-labelled second polyclonal anti-human resistin antibody is added and incubated with captured resistin for 60 minutes. After another washing, streptavidin-HRP conjugate is added. After 60 minutes incubation and the last washing step, the remaining conjugate is allowed to react with the substrate solution (TMB). The reaction is stopped by addition of acidic solution and absorbance of the resulting yellow product is measured. The absorbance is proportional to the concentration of resistin. A standard curve is constructed by plotting absorbance values against concentrations of Standards, and concentrations of unknown samples are determined using this standard curve.
Intended use
Clinical Application
Test principle

Summary of protocol
– Afify M, Sayed M, Elhammady A. Clinical utility of biochemical markers in ulcerative colitis among Egyptian patients. [Journal of American Science. 2010;6(6);
– Aknc A, Karakurt C, Gurbuz S, Elkran O, Nalbantoglu O, Kocak G, Guldur T, Yologlu S. Association of cardiac changes with serum adiponectin and resistin levels in obese and overweight children. J Cardiovasc Med (Hagerstown). 2012 Mar 23;
– Al-Mrayat M, Samarasinghe Y, Treml H, Munro N, Shotliff K, McIntosh C, Feher MD. A new cause of neuroglycopenia: “missing the point”. Diabet Med. 2004 May;21 (5):497
– Aller R, de Luis DA, Fernandez L, Calle F, Velayos B, Olcoz JL, Izaola O, Sagrado MG, Conde R, Gonzalez JM. Influence of insulin resistance and adipokines in the grade of steatosis of nonalcoholic fatty liver disease. Dig Dis Sci. 2008 Apr;53 (4):1088-92
– Aller R, De Luis DA, Izaola O, Gonzalez Sagrado M, Conde R, Alvarez T, Pacheco D, Velasco MC. Role of -55CT polymorphism of UCP3 gene on non alcoholic fatty liver disease and insulin resistance in patients with obesity. Nutr Hosp. 2010 Jul-Aug;25 (4):572-6
– Anderlova K, Dolezalova R, Housova J, Bosanska L, Haluzikova D, Kremen J, Skrha J, Haluzik M. Influence of PPAR-alpha agonist fenofibrate on insulin sensitivity and selected adipose tissue-derived hormones in obese women with type 2 diabetes. Physiol Res. 2007;56 (5):579-86
– Annaloro C, Usardi P, Airaghi L, Giunta V, Forti S, Orsatti A, Baldini M, Delle Volpe A, Lambertenghi Deliliers G. Prevalence of metabolic syndrome in long-term survivors of hematopoietic stem cell transplantation. Bone Marrow Transplant. 2008 May;41 (9):797-804
– Asgeirsson T, Zhang S, Khoo SK, Resau JH, Dujovny N, Senagore AJ. Serum adiponectin, resistin, and circulating soluble receptor for advanced glycation end products in colectomy patients. Mediators Inflamm. 2011;2011:916807
– Auguet T, Aragones G, Guiu-Jurado E, Berlanga A, Curriu M, Martinez S, Alibalic A, Aguilar C, Camara ML, Hernandez E, Ruyra X, Martin-Paredero V, Richart C. Adipo/cytokines in atherosclerotic secretomes: increased visfatin levels in unstable carotid plaque. BMC Cardiovasc Disord. 2016;16 (1):149
– Auguet T, Quintero Y, Riesco D, Morancho B, Terra X, Crescenti A, Broch M, Aguilar C, Olona M, Porras JA, Hernandez M, Sabench F, del Castillo D, Richart C. New adipokines vaspin and omentin. Circulating levels and gene expression in adipose tissue from morbidly obese women. BMC Med Genet. 2011;12:60
– Auguet T, Terra X, Porras JA, Orellana-Gavalda JM, Martinez S, Aguilar C, Lucas A, Pellitero S, Hernandez M, Del Castillo D, Richart C. Plasma visfatin levels and gene expression in morbidly obese women with associated fatty liver disease. Clin Biochem. 2013 Feb;46 (3):202-8
– Azuma K, Katsukawa F, Oguchi S, Murata M, Yamazaki H, Shimada A, Saruta T. Correlation between serum resistin level and adiposity in obese individuals. Obes Res . Aug;11(8):997-1001 (2003)
– Azuma K, Oguchi S, Matsubara Y, Mamizuka T, Murata M, Kikuchi H, Watanabe K, Katsukawa F, Yamazaki H, Shimada A, Saruta T. Novel resistin promoter polymorphisms: association with serum resistin level in Japanese obese individuals. Horm Metab Res. 2004 Aug;36 (8):564-70
– Bahr MJ, Ockenga J, Boker KH, Manns MP, Tietge UJ. Elevated resistin levels in cirrhosis are associated with the proinflammatory state and altered hepatic glucose metabolism but not with insulin resistance. Am J Physiol Endocrinol Metab . Aug;291(2):E199-206
– Bajaj M, Suraamornkul S, Hardies LJ, Pratipanawatr T, DeFronzo RA. Plasma resistin concentration, hepatic fat content, and hepatic and peripheral insulin resistance in pioglitazone-treated type II diabetic patients. Int J Obes Relat Metab Disord . Mar;60(3):350-7 (2004)
– Barac A, Campia U, Matuskey LA, Lu L, Panza JA. Effects of peroxisome proliferator-activated receptor-gamma activation with pioglitazone on plasma adipokines in nondiabetic patients with either hypercholesterolemia or hypertension. Am J Cardiol. 2008 Apr 1;101 (7):980-5
– Bergheim I, Luyendyk JP, Steele C, Russell GK, Guo L, Roth RA, Arteel GE. Metformin prevents endotoxin-induced liver injury after partial hepatectomy. J Pharmacol Exp Ther . Mar;316(3):1053-61 (2006)
– Bo S, Gambino R, Pagani A, Guidi S, Gentile L, Cassader M, Pagano GF. Relationships between human serum resistin, inflammatory markers and insulin resistance. Int J Obes (Lond) . Nov;29(11):1315-20 (2005)
– Bokarewa M, Nagaev I, Dahlberg L, Smith U, Tarkowski A. Resistin, an adipokine with potent proinflammatory properties. J Immunol. 2005 May 1;174 (9):5789-95
– BOSANSKA L, PETRAK O, ZELINKA T, MRAZ M, WIDIMSKY J Jr, HALUZIK M. The effect of pheochromocytoma treatment on subclinical inflammation and endocrine function of adipose tissue. Physiol Res. 2009;58 (3):319-25
– Broch M, Ramirez R, Auguet MT, Alcaide MJ, Aguilar C, Garcia-Espana A, Richart C. Macrophages are novel sites of expression and regulation of retinol binding protein-4 (RBP4). Physiol Res. 2010;59 (2):299-303
– Burnett MS, Devaney JM, Adenika RJ, Lindsay R, Howard BV. Cross-sectional associations of resistin, coronary heart disease, and insulin resistance. J Clin Endocrinol Metab . Jan;91(1):64-8 (2006)
– Carmina E, Orio F, Palomba S, Cascella T, Longo RA, Colao AM, Lombardi G, Lobo RA. Evidence for altered adipocyte function in polycystic ovary syndrome. Eur J Endocrinol . Mar;152(3):389-94 (2005)
– Carmina E, Orio F, Palomba S, Longo RA, Cascella T, Colao A, Lombardi G, Rini GB, Lobo RA. Endothelial dysfunction in PCOS: role of obesity and adipose hormones. Am J Med . Apr;119(4):356.e1-6 (2006)
– Chalvatzas N, Dafopoulos K, Kosmas G, Kallitsaris A, Pournaras S, Messinis IE. Effect of ovarian hormones on serum adiponectin and resistin concentrations. Fertil Steril. 2008 Mar 4;
– Chen D, Dong M, Fang Q, He J, Wang Z, Yang X. Alterations of serum resistin in normal pregnancy and pre-eclampsia. Clin Sci (Lond) . Jan;108(1):81-4 (2005)
– Chu MC, Cosper P, Nakhuda GS, Lobo RA. A comparison of oral and transdermal short-term estrogen therapy in postmenopausal women with metabolic syndrome. Fertil Steril . Dec;86(6):1669-75 (2006)
– Chu MC, Cosper P, Orio F, Carmina E, Lobo RA. Insulin resistance in postmenopausal women with metabolic syndrome and the measurements of adiponectin, leptin, resistin, and ghrelin. Am J Obstet Gynecol . Jan;194(1):100-4 (2006)
– Corpeleijn E, Feskens EJ, Jansen EH, Mensink M, Saris WH, Blaak EE. Lifestyle intervention and adipokine levels in subjects at high risk for type 2 diabetes: the Study on Lifestyle intervention and Impaired glucose tolerance Maastricht (SLIM). Diabetes Care . Dec;30(12):3125-7 (2007)
– Cortelazzi D, Corbetta S, Ronzoni S, Pelle F, Marconi A, Cozzi V, Cetin I, Cortelazzi R, Beck-Peccoz P, Spada A. Maternal and foetal resistin and adiponectin concentrations in normal and complicated pregnancies. Clin Endocrinol (Oxf) . Mar;66(3):447-53 (2007)
– De Courten BV, Degawa-Yamauchi M, Considine RV, Tataranni PA. High Serum Resistin Is Associated With an Increase in Adiposity But Not a Worsening of Insulin Resistance in Pima Indians. Diabetes . May;53(5):1279-1284 (2004)
– de Luis DA, Aller R, Gonzalez Sagrado M, Conde R, Izaola O, de la Fuente B. Genetic variation in the cannabinoid receptor gene (CNR1) (G1359A polymorphism) and their influence on anthropometric parameters and metabolic parameters under a high monounsaturated vs. high polyunsaturated fat hypocaloric diets. J Nutr Biochem. 2013 Aug;24 (8):1431-5
– de Luis DA, Aller R, Izaola O, de la Fuente B, Conde R, Sagrado MG, Primo D. Evaluation of weight loss and adipocytokines levels after two hypocaloric diets with different macronutrient distribution in obese subjects with RS9939609 gene variant. Diabetes Metab Res Rev. 2012 Aug 3;
– de Luis DA, Aller R, Izaola O, Gonzalez Sagrado M, Bellioo D, Conde R. Effects of a low-fat versus a low-carbohydrate diet on adipocytokines in obese adults. Horm Res . 67(6):296-300 (2007)
– de Luis DA, Aller R, Izaola O, Gonzalez Sagrado M, Conde R, de la Fuente B, Perez Castrillon JL. Relationship of insulin resistance and adipocytokines on serum alanine aminotransferase in presurgical morbid obese patients. Eur Rev M

