Midkine Human ELISA
Midkine (MK, also called neurite growth promoting factor 2, NEGF-2), a product of a retinoic acid responsive gene, is a secreted 13 kDa protein belonging to the family of heparin binding growth/differentiation factors. MK shares 45% sequence identity with another member of this family called Pleiotrophin (HB-GAM). Midkine is composed of two domains held together by disulfide linkages. The C-terminally located domain contains two heparin binding sites and is usually responsible for midkine activity. Part of the MK activity is enhanced by dimerization of MK.
Midkine has been found in vertebrates from human to zebrafish and is most strongly expressed in midgestation. In the adult MK expression is restricted. In addition to normal development, MK is also involved in the pathogenesis of diseases, e.g. inflammatory diseases, human carcinomas such as esophageal, stomach, colon, pancreatic, thyroid, lung, urinary, hepatocellular, neuroblastoma, glioblastoma, Wilm´s tumor etc. High MK levels are associated with poor prognosis in some types of cancer. The increased expression in many carcinomas indicates that MK can be applied to the diagnosis of malignancy. Midkine is expressed during the reparative stage of bone fractures, also supresses infection of cells by some viruses including HIV. Anti-apoptotic and cell protecting activity of midkine makes it promising in therapy.
The RD191042200R Human Midkine ELISA is a sandwich enzyme immunoassay for the quantitative measurement of human midkine in serum, plasma (citrate, heparin).
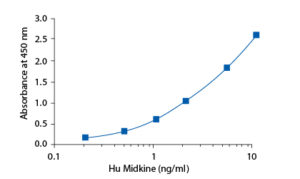
In the BioVendor Human Midkine ELISA, standards, quality controls and samples are incubated in microplate wells pre-coated with polyclonal anti-human midkine antibody. After 120 minutes incubation at 37°C and washing, biotin labelled polyclonal anti-human midkine antibody is added and incubated with captured midkine for 60 minutes. After another washing, streptavidin–HRP conjugate is added. After 30 minutes incubation and the last washing step, the remaining HRP conjugate is allowed to react with the substrate solution (TMB). The reaction is stopped by addition of acidic solution and absorbance of the resulting yellow product is measured. The absorbance is proportional to the concentration of midkine. A standard curve is constructed by plotting absorbance values against concentrations of standards, and concentrations of unknown samples are determined using this standard curve.
Intended use
Clinical Application
Test principle

Summary of protocol
– Hodeib H, ELshora O, Selim A, Sabry NM, El-Ashry HM. Serum Midkine and Osteopontin Levels as Diagnostic Biomarkers of Hepatocellular Carcinoma. Electron Physician. 2017 Jan 25;9(1):3492-3498. doi: 10.19082/3492
– Jia HL, Ye QH, Qin LX, Budhu A, Forgues M, Chen Y, Liu YK, Sun HC, Wang L, Lu HZ, Shen F, Tang ZY, Wang XW. Gene expression profiling reveals potential biomarkers of human hepatocellular carcinoma. Clin Cancer Res . Feb 15;13(4):1133-9 (2007)
– Krzystek-Korpacka M, Neubauer K, Matusiewicz M. Circulating midkine in Crohn’s disease: clinical implications. Inflamm Bowel Dis. 2010 Feb;16 (2):208-15
– Krzystek-Korpacka M, Neubauer K, Matusiewicz M. Clinical relevance of circulating midkine in ulcerative colitis. Clin Chem Lab Med. 2009;47 (9):1085-90
– Malyszko J, ORCID: 0000-0001-8701-8171, Bachorzewska-Gajewska H, Koc-Zorawska E, Malyszko JS, Kobus G, Dobrzycki S. Midkine: a novel and early biomarker of contrast-induced acute kidney injury in patients undergoing percutaneous coronary interventions. Biomed Res Int. 2015;2015:879509
– Tong Y, Mentlein R, Buhl R,Heinz-Hermann H, Krause J, Mehdorn HM, Held-Feindt J. Overexpression of midkine contributes to anti-apoptotic effects in human meningiomas. Journal of Neurochemistry . Feb;100(4):1097-107 (2007)
– Zhang YW, Denham J, Thies RS. Oligodendrocyte progenitor cells derived from human embryonic stem cells express neurotrophic factors. Stem Cells Dev . Dec;15(6):943-52 (2006)

