Synuclein Gamma Human ELISA
Synucleins are small, highly conserved, soluble proteins expressed primarily in neural tissue and in certain tumors. The synuclein family consists of three members: α-, β-, and γ-synuclein present only in vertebrates [1].
Synuclein-γ (SNCG) is a human gene localized at 10q23.2023.3. SNCG cDNA is about 5 kb in length and comprised of five exons that translate into a 17 kDa cytoplasmic protein consisting of 127 amino acids [2].
Synuclein-γ (SNCG) is implicated in many biochemical functions, but its exact role is not completely understood. As this aggregation-prone protein is highly expressed in healthy neuronal cells its involvement in the neurodegenerative diseases has been extensively studied [3]. Some publication suggest that γ-synuclein may change its intracellular localization in response to stress and make appropriate alterations in the gene expression pattern [4]. SNCG can function like chaperone protein and stimulates hormoneresponsive mammary tumorigenesis [5]. The abnormal expression and elevated levels of γ-synuclein protein has been demonstrated in many different malignant diseases [6, 7].
Recently, a clinical study showed that the multi-marker test combining cytology and urinary levels of midkine and synuclein-γ can improve the detection of bladder cancer during the primary diagnostics and also during monitoring of patients with non-muscle-invasive bladder cancer [8].
The RD191397200CS Human Synuclein Gamma ELISA is a sandwich enzyme immunoassay for the quantitative measurement of human synuclein gamma (SNCG).
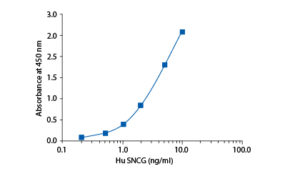
In the BioVendor Human Synuclein Gamma ELISA, standards and samples are incubated in microplate wells pre-coated with anti-human SNCG antibody. After 60 minutes incubation and washing, biotin-labelled polyclonal anti-human SNCG antibody is added and incubated with captured SNCG for 60 minutes. After another washing, streptavidin-horseradish peroxidase conjugate is added. After 30 minutes incubation and the last washing step, the remaining conjugate is allowed to react with the substrate solution (TMB). The reaction is stopped by addition of acidic solution and absorbance of the resulting yellow product is measured. The absorbance is proportional to the concentration of SNCG. A standard curve is constructed by plotting absorbance values against concentrations of standards, and concentrations of unknown samples are determined using this standard curve.
Intended use
Clinical Application
Test principle
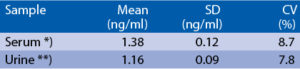
Precision
Intra-assay (Within-Run) (n=8)
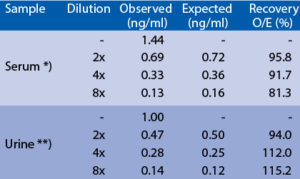
Serum or urine samples were spiked with different amounts of human SNCG and assayed. *) Sample from patient with craniotrauma Serum or urine samples were serially diluted with Dilution Buffer and assayed. *) Sample from patient with craniotrauma **) Sample from patient with bladder cancer
Spiking recovery

**) Sample from patient with bladder cancerLinearity
Summary of protocol
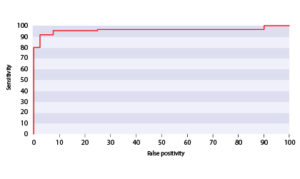
Diagnosis and monitoring of patients with non-muscle-invasive bladder cancer (NMIBC) Study group definition: 49 healthy controls, 61 patients with a history of NMIBC without current disease, and 114 patients with bladder cancer. The results of the study showed that the multi-marker test combining cytology and urinary levels of midkine and synuclein gamma can improve the detection of bladder cancer during the primary diagnostics and also during monitoring of patients with NMIBC with clinical specificity 97.5% and sensitivity 91.8% as shown on ROC curve.
Clinical Relevance
– Soukup V, Kalousova M, Capoun O, Sobotka R, Breyl Z, Pesl M, Zima T, Hanus T. Panel of Urinary Diagnostic Markers for Non-Invasive Detection of Primary and Recurrent Urothelial Urinary Bladder Carcinoma. Urol Int. 2015;95 (1):56-64

