Syndecan-1 (CD138) Human ELISA
Syndecans are a transmembrane protein family within the heparin sulphate proteoglycan group that interact with many different molecules of the immune system through their heparin sulphate chains. The mammalian syndecan family consists of 4 proteins; syndecan 1 to 4 each encoded by very distinct genes. In adult tissues syndecan 1 (CD138) is predominantly expressed by epithelial cells and plasma cells (both normal and malignant) and currently considered the most reliable surface marker for plasma cells. In addition CD138 is also expressed on pre and immature B cells however this is regulated by IL-6 and LPS stimulation. Syndecan 1 has previously been shown to participate in cell to cell interactions, organ development, vessel formation and tissue regeneration following injury. CD138 is regularly cleaved from the membrane and as a consequence high levels of soluble CD138 are found in the blood, which can be easily detected using a CD138 specific ELISA. Via its heparin sulphate chains CD138 binds to and modulates the activity of a wide range of molecules involve in inflammation including chemokines, growth factors, selectins and other adhesion molecules. CD138 can also act as a receptor for collagen, fibronectin, thromobospondin and tenascin therefore involved in cell matrix adhesion. CD138 has been shown to mediate the binding of myeloma cells to type I collagen, and inhibits tumour cell invasion into collagen gels. As CD138 has been shown to have important effects on tumour cell growth, survival, adhesion and invasion syndecan-1 may be an important regulator in cancer biology.
Research topic
Cell surface proteins (sCD), Oncology
Type
Sandwich ELISA, Biotin-labelled antibody
Applications
Serum, Plasma, Cell culture supernatant
Sample Requirements
100 µl/well
Storage/Expiration
Store the complete kit at 2–8°C. Under these conditions, the kit is stable until the expiration date (see label on the box).
Calibration Curve
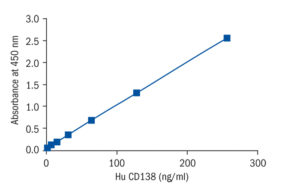
Calibration Range
8–256 ng/ml
Limit of Detection
< 2.56 ng/ml
– Annecke T, Chappell D, Chen C, Jacob M, Welsch U, Sommerhoff CP, Rehm M, Conzen PF, Becker BF. Sevoflurane preserves the endothelial glycocalyx against ischaemia-reperfusion injury. Br J Anaesth. 2010 Apr;104 (4):414-21
– Bruegger D, Jacob M, Rehm M, Loetsch M, Welsch U, Conzen P, Becker BF. Atrial natriuretic peptide induces shedding of endothelial glycocalyx in coronary vascular bed of guinea pig hearts. Am J Physiol Heart Circ Physio. 2005 Nov;289 (5):H1993-9
– Bruegger D, Rehm M, Abicht J, Paul JO, Stoeckelhuber M, Pfirrmann M, Reichart B, Becker BF, Christ F. Shedding of the endothelial glycocalyx during cardiac surgery: on-pump versus off-pump coronary artery bypass graft surgery. J Thorac Cardiovasc Surg. 2009 Dec;138 (6):1445-7
– Celie JW, Reijmers RM, Slot EM, Beelen RH, Spaargaren M, Ter Wee PM, Florquin S, van den Born J. Tubulointerstitial heparan sulfate proteoglycan changes in human renal diseases correlate with leukocyte influx and proteinuria. Am J Physiol Renal Physiol. 2008 Jan;294 (1):F253-63
– Chappell D, Jacob M, Hofmann-Kiefer K, Rehm M, Welsch U, Conzen P, Becker BF. Antithrombin reduces shedding of the endothelial glycocalyx following ischaemia/reperfusion. Cardiovasc Res. 2009 Jul 15;83 (2):388-96
– Iwata H, Matsuo K, Takeuchi K, Kishi Y, Murashige N, Kami M. High incidences of malignant lymphoma in patients infected with hepatitis B or hepatitis C virus. Haematologica. 2004 Mar;89 (3):368-70
– Janosi J, Sebestyen A, Mikala G, Nemeth J, Kiss Z, Valyi-Nagy I. Soluble syndecan-1 levels in different plasma cell dyscrasias and in different stages of multiple myeloma. Haematologica. 2004 Mar;89 (3):370-1
– Kliment CR, Englert JM, Gochuico BR, Yu G, Kaminski N, Rosas I, Oury TD. Oxidative stress alters syndecan-1 distribution in lungs with pulmonary fibrosis. J Biol Chem. 2009 Feb 6;284 (6):3537-45
– Mahtouk K, Hose D, Raynaud P, Hundemer M, Jourdan M, Jourdan E, Pantesco V, Baudard M, De Vos J, Larroque M, Moehler T, Rossi JF, Reme T, Goldschmidt H, Klein B. Heparanase influences expression and shedding of syndecan-1, and its expression by the bone marrow environment is a bad prognostic factor in multiple myeloma. Blood. 2007 Jun 1;109 (11):4914-23
– Molica S, Vitelli G, Mirabelli R, Digiesu G, Giannarelli D, Cuneo A, Ribatti D, Vacca A. Serum levels of syndecan-1 in B-cell chronic lymphocytic leukemia: correlation with the extent of angiogenesis and disease-progression risk in early disease. Leuk Lymphoma. 2006 Jun;47 (6):1034-40
– Rehm M, Bruegger D, Christ F, Conzen P, Thiel M, Jacob M, Chappell D, Stoeckelhuber M, Welsch U, Reichart B, Peter K, Becker BF. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation. 2007 Oct 23;116 (17):1896-906
– Schaar CG, Vermeer HJ, Wijermans PW, Huisman W, le Cessie S, Kluin-Nelemans HC. Serum syndecan-1 in patients with newly diagnosed monoclonal proteinemia. Haematologica. 2005 Oct;90 (10):1437-8
– Seidel C, Sundan A, Hjorth M, Turesson I, Dahl IM, Abildgaard N, Waage A, Borset M. Serum syndecan-1: a new independent prognostic marker in multiple myeloma. Blood. 2000 Jan 15;95 (2):388-92
– Snoeijs MG, Vink H, Voesten N, Christiaans MH, Daemen JW, Peppelenbosch AG, Tordoir JH, Peutz-Kootstra CJ, Buurman WA, Schurink GW, van Heurn LW. Acute ischemic injury to the renal microvasculature in human kidney transplantation. Am J Physiol Renal Physiol. 2010 Nov;299 (5):F1134-40
– Theocharis AD, Seidel C, Borset M, Dobra K, Baykov V, Labropoulou V, Kanakis I, Dalas E, Karamanos NK, Sundan A, Hjerpe A. Serglycin constitutively secreted by myeloma plasma cells is a potent inhibitor of bone mineralization in vitro. J Biol Chem. 2006 Nov 17;281 (46):35116-28
– Thiara AS, Andersen VY, Videm V, Mollnes TE, Svennevig K, Hoel TN, Fiane AE. Comparable biocompatibility of Phisio- and Bioline-coated cardiopulmonary bypass circuits indicated by the inflammatory response. Perfusion. 2010 Jan;25 (1):9-16
– Thiara AS, Mollnes TE, Videm V, Andersen VY, Svennevig K, Kolset SO, Fiane AE. Biocompatibility and pathways of initial complement pathway activation with Phisio- and PMEA-coated cardiopulmonary bypass circuits during open-heart surgery. Perfusion. 2011 Mar;26 (2):107-14

