Surfactant Protein D Human ELISA
Human Surfactant Protein D (SP-D) is a member of the collageneous subfamily of glycoproteins and calcium-dependent lectins (collectins). SP-D is a homotrimeric protein consisting of three 43kDa units that are bonded at their Ntermini. Most preparations of SP-D contain predominantly dodecamers (four trimeric subunits), but also higher multimers have been observed. Each unit consists of at least four discrete structural domains: a short N-terminal domain; a relatively long collagenous domain, a short amphipathic connecting peptide, and a C-terminal, C- type lectin carbohydrate recognition domain (CRD).
SP-D is synthesized and secreted by two types of non-ciliated epithelial cells in the peripheral airway, alveolar type II cells and Clara cells. It is also expressed by various epithelial cells in the gastrointestinal and genitourinary tracts and placenta. In the lungs, SP-D participates in the innate response to inhaled microorganisms and organic antigens. SP-D acts by aggregating bacteria and viruses, leukocyte function and stimulating an allergenic response. SP-D binds to the surface glycoconjugates of various microorganisms (eg, influenza virus, HIV, HSV, RSV, Mycoplasma pneumoniae) and the oligosaccharides associated with the surface of numerous organic antigens and enhances their phagocytosis. Studies have shown that SP-D binds to T cells, thus inhibiting their proliferation. SP-D also binds with inflammatory ligands via protein-protein and protein-carbohydrate interactions that are effective in reducing specific inflammation. In addition, SP-D binds to apoptotic cells and stimulates their phagocytosis by macrophages governed by mechanisms dependent and CD91 calreticulin.
Given that SP-D together with SP-A affects the reactivity of immune cells, their presence in the endometrium and placenta plays an important role in protection against bacteria and toxins during pregnancy. Reduced levels of all components of pulmonary surfactant, including SP-D, has been linked to premature birth.
Disturbance of pulmonary surfactant is in many cases the reason for collapse of the lungs and is also associated with many pulmonary diseases. All types of chronic lung disease is characterized by pathologically altered levels in lung tissue (fibrosis and emphysema). Studies have shown that expression of SP-D is associated with many pulmonary diseases: cystic fibrosis, acute interstitial pneumonia (ARDS), chronic obstructive pulmonary disease, asthma, bronchopulmonary dysplasia, alveolar capillary dysplasia, alveolar proteinase and tuberculosis. Clinical application and areas of investigation: Cystic fibrosis, Acute interstitial pneumonia (ARDS), Chronic obstructive pulmonary disease, Asthma, Bronchopulmonary dysplasia, Alveolar capillary dysplasia and alveolar proteinase, Immune response, infection and inflammation
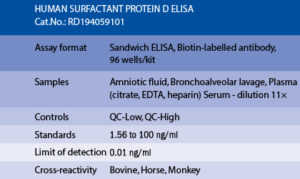
The RD194059101 Human Surfactant Protein D ELISA is a sandwich enzyme immunoassay for the quantitative measurement of human surfactant protein D in serum, plasma bronchoalveolar lavage fluid and amniotic fluid.
In the BioVendor Human Surfactant Protein D ELISA, Standards, Quality Controls and samples are incubated in microplate wells pre-coated with monoclonal anti-human surfactant protein D antibody. After 120 minutes incubation and washing, biotin labelled monoclonal antihuman SP-D antibody is added and incubated with the captured SP-D for 60 minutes. After another washing, streptavidin-HRP conjugate is added. After 60 minutes incubation and the last washing step, the remaining HRP conjugate is allowed to react with the substrate solution (TMB). The reaction is stopped by addition of acidic solution and absorbance of the resulting yellow product is measured. The absorbance is proportional to the concentration of surfactant protein D. A standard curve is constructed by plotting absorbance values against concentrations of standards, and concentrations of unknown samples are determined using this standard curve.
Intended use
Clinical Application
Test principle

Summary of protocol
– Alexis NE, Lay JC, Haczku A, Gong H, Linn W, Hazucha MJ, Harris B, Tal-Singer R, Peden DB. Fluticasone propionate protects against ozone-induced airway inflammation and modified immune cell activation markers in healthy volunteers. Environ Health Perspect. 2008 Jun;116 (6):799-805
– Anderson JT, Zeng M, Li Q, Stapley R, Moore DR 2nd, Chenna B, Fineberg N, Zmijewski J, Eltoum IE, Siegal GP, Gaggar A, Barnes S, Velu SE, Thannickal VJ, Abraham E, Patel RP, Lancaster JR Jr, Chaplin DD, Dransfield MT, Deshane JS. Elevated levels of NO are localized to distal airways in asthma. Free Radic Biol Med. 2011 Jun 1;50 (11):1679-88
– Aul R, Armstrong J, Duvoix A, Lomas D, Hayes B, Miller BE, Jagger C, Singh D. Inhaled LPS challenges in smokers: a study of pulmonary and systemic effects. Br J Clin Pharmacol. 2012 Dec;74 (6):1023-32
– Barlo NP, van Moorsel CH, Ruven HJ, Zanen P, van den Bosch JM, Grutters JC. Surfactant protein-D predicts survival in patients with idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung . 2009 Jul;26 (2):155-61
– Bejvl I, Weseslindtner L, Strassl R, Jaksch P, Kundi M, Klepetko W, Puchhammer-Stockl E. Analysis of plasma surfactant protein D levels in lung transplant recipients. Transpl Infect Dis. 2013 Dec;15 (6):645-51
– Bonlokke JH, Riddervold IS, Gronborg TK, Skogstrand K, Hougaard DM, Barregard L, Sigsgaard T. Systemic effects of wood smoke in a short-term experimental exposure study of atopic volunteers. J Occup Environ Med. 2014 Feb;56 (2):177-83
– Bratcher PE, Gaggar A. Factors influencing the measurement of plasma/serum surfactant protein d levels by ELISA. PLoS One. 2014;9 (11):e111466
– Bullone M, de Lagarde M, Vargas A, Lavoie JP. Serum Surfactant Protein D and Haptoglobin as Potential Biomarkers for Inflammatory Airway Disease in Horses. J Vet Intern Med. 2015 Nov-Dec;29 (6):1707-11
– Daneshzadeh Tabrizi R, Bernard A, Thommen AM, De Winter F, Oppliger A, Hilfiker S, Tschopp A, Hotz P. Surfactant protein-D and exposure to bioaerosols in wastewater and garbage workers. Int Arch Occup Environ Health. 2010 Dec;83 (8):879-86
– Delgado C, Krotzsch E, Jimenez-Alvarez LA, Ramirez-Martinez G, Marquez-Garcia JE, Cruz-Lagunas A, Moran J, Hernandez C, Sierra-Vargas P, Avila-Moreno F, Becerril C, Montano M, Banales-Mendez JL, Zuniga J, Buendia-Roldan I. Serum surfactant protein D (SP-D) is a prognostic marker of poor outcome in patients with A/H1N1 virus infection. Lung. 2015 Feb;193 (1):25-30
– Duda I, Grzybowska K, Jedrzejowska-Szypulka H, Lewin-Kowalik J. The sitting position during neurosurgical procedures does not influence serum biomarkers of pulmonary parenchymal injury. BMC Surg. 2012;12:24
– El-Deek SE, Makhlouf HA, Saleem TH, Mandour MA, Mohamed NA. Surfactant protein D, soluble intercellular adhesion molecule-1 and high-sensitivity C-reactive protein as biomarkers of chronic obstructive pulmonary disease. Med Princ Pract. 2013;22 (5):469-74
– Ellingsen DG, Ulvestad B, Andersson L, Barregard L. Pneumoproteins and inflammatory biomarkers in asphalt pavers. Biomarkers. 2010 Sep;15 (6):498-507
– Engstrom G, Lindberg C, Gerhardsson de Verdier M, Nihlen U, Anderson M, Svartengren M, Forsman-Semb K. Blood biomarkers and measures of pulmonary function–a study from the Swedish twin registry. Respir Med. 2012 Sep;106 (9):1250-7
– Erpenbeck VJ, Ziegert M, Cavalet-Blanco D, Martin C, Baelder R, Glaab T, Braun A, Steinhilber W, Luettig B, Uhlig S, Hoymann HG, Krug N, Hohlfeld JM. Surfactant protein D inhibits early airway response in Aspergillus fumigatus-sensitized mice. Clin Exp Allergy. 2006 Jul;36 (7):930-40
– Fernandez-Luna A, Gallardo L, Plaza-Carmona M, Garcia-Unanue J, Sanchez-Sanchez J, Felipe JL, Burillo P, Ara I. Respiratory function and changes in lung epithelium biomarkers after a short-training intervention in chlorinated vs. ozone indoor pools. PLoS One. 2013;8 (7):e68447
– Fernandez-Real JM, Valdes S, Manco M, Chico B, Botas P, Campo A, Casamitjana R, Delgado E, Salvador J, Fruhbeck G, Mingrone G, Ricart W. Surfactant protein d, a marker of lung innate immunity, is positively associated with insulin sensitivity. Diabetes Care. 2010 Apr;33 (4):847-53
– Font-Ribera L, Kogevinas M, Zock JP, Gomez FP, Barreiro E, Nieuwenhuijsen MJ, Fernandez P, Lourencetti C, Perez-Olabarria M, Bustamante M, Marcos R, Grimalt JO, Villanueva CM. Short-term changes in respiratory biomarkers after swimming in a chlorinated pool. Environ Health Perspect. 2010 Nov;118 (11):1538-44
– Foreman MG, Kong X, DeMeo DL, Pillai SG, Hersh CP, Bakke P, Gulsvik A, Lomas DA, Litonjua AA, Shapiro SD, Tal-Singer R, Silverman EK. Polymorphisms in surfactant protein-D are associated with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2011 Mar;44 (3):316-22
– Gargiulo P, Banfi C, Ghilardi S, Magri D, Giovannardi M, Bonomi A, Salvioni E, Battaia E, Filardi PP, Tremoli E, Agostoni P. Surfactant-derived proteins as markers of alveolar membrane damage in heart failure. PLoS One. 2014;9 (12):e115030
– Gaunsbaek MQ, Kjeldsen AD, Svane-Knudsen V, Henriksen ML, Hansen S. Surfactant proteins A, B, C and D in the human nasal airway: associated with mucosal glands and ciliated epithelium but absent in fluid-phase secretions and mucus. ORL J Otorhinolaryngol Relat S. 2014;76 (5):288-301
– Gil HW, Hong JR, Park JH, Seo YS, Yang JO, Lee EY, Hong SY. Plasma surfactant D in patients following acute paraquat intoxication. Clin Toxicol (Phila). 2007 Jun-Aug;45 (5):463-7
– Glas J, Beynon V, Bachstein B, Steckenbiller J, Manolis V, Euba A, Muller-Myhsok B, Folwaczny M. Increased plasma concentration of surfactant protein D in chronic periodontitis independent of SFTPD genotype: potential role as a biomarker. Tissue Antigens. 2008 Jul;72 (1):21-8
– Guzel A, Karadag A, Okuyucu A, Alacam H, Kucuk Y. The evaluation of serum surfactant protein D (SP-D) levels as a biomarker of lung injury in tuberculosis and different lung diseases. Clin Lab. 2014;60 (7):1091-8
– Hant FN, Ludwicka-Bradley A, Wang HJ, Li N, Elashoff R, Tashkin DP, Silver RM. Surfactant protein D and KL-6 as serum biomarkers of interstitial lung disease in patients with scleroderma. J Rheumatol. 2009 Apr;36 (4):773-80
– Heldal KK, Barregard L, Larsson P, Ellingsen DG. Pneumoproteins in sewage workers exposed to sewage dust. Int Arch Occup Environ Health. 2013 Jan;86 (1):65-70
– Hill J, Heslop C, Man SF, Frohlich J, Connett JE, Anthonisen NR, Wise RA, Tashkin DP, Sin DD. Circulating surfactant protein-D and the risk of cardiovascular morbidity and mortality. Eur Heart J. 2011 Aug;32 (15):1918-25
– Hodge S, Hodge G, Jersmann H, Matthews G, Ahern J, Holmes M, Reynolds PN. Azithromycin improves macrophage phagocytic function and expression of mannose receptor in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008 Jul 15;178 (2):139-48
– Holz O, Tan L, Schaumann F, Muller M, Scholl D, Hidi R, McLeod A, Krug N, Hohlfeld JM. Inter- and intrasubject variability of the inflammatory response to segmental endotoxin challenge in healthy volunteers. Pulm Pharmacol Ther. 2015 Dec;35:50-9
– Ju CR, Liu W, Chen RC. Serum surfactant protein D: biomarker of chronic obstructive pulmonary disease. Dis Markers. 2012;32 (5):281-7
– Karottki DG, Spilak M, Frederiksen M, Jovanovic Andersen Z, Madsen AM, Ketzel M, Massling A, Gunnarsen L, Moller P, Loft S. Indoor and outdoor exposure to ultrafine, fine and microbiologically derived particulate matter related to cardiovascular and respiratory effects in a panel of elderly urban citizens. Int J Environ Res Public Healt. 2015 Feb;12 (2):1667-86
– Kor DJ, Carter RE, Park PK, Festic E, Banner-Goodspeed VM, Hinds R, Talmor D, Gajic O, Ware LB, Gong MN. Effect of Aspirin on Development of ARDS in At-Risk Patients Presenting to the Emergency Department: The LIPS-A Randomized Clinical Trial. JAMA. 2016 Jun 14;315 (22):2406-14
– Kucejko W, Chyczewska E, Naumnik W, Ossolinska M. Concentration of surfactant protein D, Clara cell protein CC-16 and IL-10 in bronchoalveolar lavage (BAL) in patients with sarcoidosis, hypersensivity pneumonitis and idiopathic pulmonary fibrosis. Folia Histochem Cytobiol. 2009;47 (2):225-30 – Kunisaki KM, Quick H, Baker JV.

