sTfR Human ELISA (soluble Transferrin Receptor)
The transferrin receptor (TfR) is the gateway for transferrin-bound-iron entering all body cells. TfR is abundant on the surface of many newly formed cells, but the erythroid marrow cells account for 70 to 80 % of the total body TfR content. The soluble (or serum) transferrin receptor (sTfR) is a circulating truncated form of the membrane receptor protein; it is an 85 kDa glycoprotein forming in serum a 320 kDa complex with diferric transferrin. The serum sTfR concentration reflects the total body mass of cellular transferrin receptor. Anaemias associated with enhanced erythropoiesis and iron deficiency result in an elevation in the sTfR values. Elevation of the soluble transferrin receptor may be also caused by haemolytic anaemia, polycythaemia and thalassemia while aplastic anaemia and chronic renal failure may result in its decrease. The most important clinical use of the sTfR determination is in the differential diagnosis between iron deficiency anaemia and the anaemia of chronic disease.
The RD194011100 Human sTfR ELISA is a sandwich enzyme immunoassay for the quantitative measurement of human soluble transferrin receptor in serum and plasma.
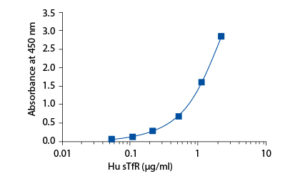
In the BioVendor Human sTfR ELISA, standards, quality controls and samples are incubated in microplate wells pre-coated with monoclonal anti-human sTfR antibody. After 60 minutes incubation and washing, monoclonal anti-human sTfR antibody, conjugated with horseradish peroxidase (HRP) is added to the wells and incubated for 60 minutes with captured sTfR. Following another washing step, the remaining HRP conjugate is allowed to react with the substrate solution (TMB). The reaction is stopped by addition of acidic solution and absorbance of the resulting yellow product is measured. The absorbance is proportional to the concentration of sTfR. A standard curve is constructed by plotting absorbance values against concentrations of standards, and concentrations of unknown samples are determined using this standard curve.
Intended use
Clinical Application
Test principle

Summary of protocol
– Dierschke K, Isaxon C, Andersson UB, Assarsson E, Axmon A, Stockfelt L, Gudmundsson A, Jönsson BA, Kåredal M, Löndahl J, Pagels J, Wierzbicka A, Bohgard M, Nielsen J. Acute respiratory effects and biomarkers of inflammation due to welding-derived nanoparticle aggregates. Int Arch Occup Environ Health. 2017 Mar 3. doi: 10.1007/s00420-017-1209-z
– El-Gendy Fady, El-Hawy MA, Rizk MS, El-Hefnawy SM, Mahmoud MZ. Value of Soluble Transferrin Receptors and sTfR/log Ferritin in the Diagnosis of Iron Deficiency Accompanied by Acute Infection. 25 May 2017. 10.1007/s12288-017-0836-6.
– Fernandez-Real JM, Izquierdo M, Moreno-Navarrete JM, Gorostiaga E, Ortega F, Martinez C, Idoate F, Ricart W, Ibanez J. Circulating soluble transferrin receptor concentration decreases after exercise-induced improvement of insulin sensitivity in obese individuals. Int J Obes (Lond). 2009 Jul;33 (7):768-74
– Fernandez-Real JM, Moreno JM, Lopez-Bermejo A, Chico B, Vendrell J, Ricart W. Circulating soluble transferrin receptor according to glucose tolerance status and insulin sensitivity. Diabetes Care . Mar;30(3):604-8 (2007)
– Jafari SM, Heidari G, Nabipour I, Amirinejad R, Assadi M, Bargahi A, Akbarzadeh S, Tahmasebi R, Sanjdideh Z. Serum retinol levels are positively correlated with hemoglobin concentrations, independent of iron homeostasis: a population-based study. Nutr Res. 2013 Apr;33 (4):279-85
– Khambalia A, O’Connor DL, Zlotkin S. Periconceptional iron and folate status is inadequate among married, nulliparous women in rural Bangladesh. J Nutr. 2009 Jun;139 (6):1179-84
– Menon KC, Ferguson EL, Thomson CD, Gray AR, Zodpey S, Saraf A, Das PK, Pandav CS, Skeaff SA. Iron status of pregnant Indian women from an area of active iron supplementation. Nutrition. 2014 Mar;30 (3):291-6
– Mojiminiyi OA, Marouf R, Abdella NA. Body iron stores in relation to the metabolic syndrome, glycemic control and complications in female patients with type 2 diabetes. Nutr Metab Cardiovasc Dis. 2008 Oct;18 (8):559-66
– Rybka J, Kedziora-Kornatowska K, Banas-Lezanska P, Majsterek I, Carvalho LA, Cattaneo A, Anacker C, Kedziora J. Interplay between the pro-oxidant and antioxidant systems and proinflammatory cytokine levels, in relation to iron metabolism and the erythron in depression. Free Radic Biol Med. 2013 Oct;63:187-94
– Sharma JB, Bumma SD,Saxena R,Kumar S,Roy KK,Singh N,Vanamail P. Cross sectional, comparative study of serum erythropoietin, transferrin receptor, ferritin levels and other hematological indices in normal pregnancies and iron deficiency anemia during pregnancy. EJOG. May 2016;:5
– Shinzato T, Abe K, Furusu A, Harada T, Shinzato K, Miyazaki M, Kohno S. Serum pro-hepcidin level and iron homeostasis in Japanese dialysis patients with erythropoietin (EPO)-resistant anemia. Med Sci Monit. 2008 Sep;14 (9):CR431-7
– Skarpańska-Stejnborn A, Basta P, Trzeciak J, Michalska A, Kafkas ME, Woitas-Ślubowska D. Effects of cranberry (Vaccinum macrocarpon) supplementation on iron status and inflammatory markers in rowers. J Int Soc Sports Nutr. 2017 Feb 28;14:7. doi: 10.1186/s12970-017-0165-z
– Stefanova KI, Delcheva GT, Maneva AI, Batalov AZ, Geneva-Popova MG, Karalilova RV, Simitchiev KK. Pathobiochemical Mechanisms Relating Iron Homeostasis to Parameters of Inflammatory Activity and Autoimmune Disorders in Rheumatoid Arthritis. Folia Med (Plovdiv). 2016 Dec 1;58(4):257-263. doi: 10.1515/folmed-2016-0040

