Sodium Assay
Liquid Stable Enzymatic Sodium Assay is intended for the quantitative in vitro determination of sodium in serum.
Sodium Assay has good correlation with the ISE method and has a linear method between sodium concentrations of 80 and 180 mmole/L (184 and 414 mg/dL). The assay has CV’s% of <2% with a correlation coefficient of 0.98, slope of 1.05 and a y intercept of -2.23. The following substances normally present in serum produced less than 10% deviation at the listed concentrations: NH4Cl at 1.5 mM, KPi at 2.0 mM, CaCl2 at 7.5 mM, KCl at 10 mM, CuCl2 at 0.5 mM, ZnCl2 at 0.5 mM, FeCl3at 0.5 mM, Glucose at 5 mM, ascorbate 10 mM, bilirubin at 40 mg/dL, bilirubin conjugate 40 mg/dL, hemoglobin 500 mg/dL, and triglyceride 1000 mg/dL.
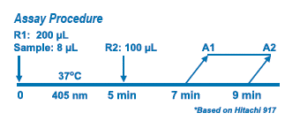
Sodium is determined enzymatically via sodium-dependent β- galactosidase activity with ONPG as the substrate. The absorbance at 405 nm of the product O-nitrophenyl is proportional to the sodium concentration.