Prostaglandin D Synthase (Lipocalin-type) Human ELISA
Lipocalin – type Prostaglandin D synthase (Beta – Trace Protein, BTP) catalyzes the conversion of PGH2 to PGD2, a prostaglandin involved in smooth muscle contraction/relaxation and a potent inhibitor of platelet aggregation. Involved in a variety of CNS functions, such as sedation, NREM (non rapid eye movement) sleep and PGE2-induced allodynia, and may have an anti-apoptotic role in oligodendrocytes. Binds small non-substrate lipophilic molecules, including biliverdin, bilirubin, retinal, retinoic acid and thyroid hormone, and may act as a scavenger for harmful hydrophopic molecules and as a secretory retinoid and thyroid hormone transporter. Possibly involved in development and maintenance of the blood-brain, blood-retina, blood-aqueous humor and blood-testis barrier. It is likely to play important roles in both maturation and maintenance of the central nervous system and male reproductive system.It has been proposed that the urinary and serum levels may provide a sensitive indicator of renal damage in diabetes mellitus and hypertension. Elevated levels in the coronary circulation may also be associated with angina. Changes in charge and molecular weight microheterogeneity, due to modification of the N-linked oligosaccharides, may be associated with neurodegenerative disease and multiple sclerosis. Detected in meningioma but not in other brain tumors and may be considered a specific cell marker for meningioma.
Features
- European Union and rest of the world: for research use only.
- The total assay time is less than 3 hours
- The kit measures Beta-trace Protein in serum and plasma (EDTA, citrate, heparin).
- Assay format is 96 wells.
- Quality Controls are human serum based. No animal sera are used.
- Standard is purified native protein based.
- Components of the kit are provided ready to use or concentrated.
Research topic
Neural tissue markers, Renal disease
Type
Sandwich ELISA, HRP-labelled antibody
Applications
Serum, Urine, Cerebrospinal fluid, Plasma
Sample Requirements
100 μl/well
Storage/Expiration
Store the complete kit at 2–8°C. Under these conditions, the kit is stable until the expiration date (see label on the box).
Calibration Curve
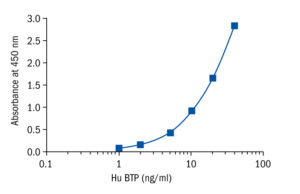
Calibration Range
1–40 ng/ml
Limit of Detection
0.5 ng/ml
Intra-assay (Within-Run)
n = 8; CV = 4.1%
Inter-assay (Run-to-Run)
n = 6; CV = 4.2%
Spiking Recovery
92,10%
Dilutation Linearity
93,10%
Crossreactivity
bovine Non-detectable cat Non-detectable dog Non-detectable goat Non-detectable hamster Non-detectable horse Non-detectable mouse Non-detectable pig Non-detectable rabbit Non-detectable rat Non-detectable sheep Non-detectable chicken Not tested human Yes monkey Yes (recommended dilution 1:50)
– Bellei E, Monari E, Bergamini S, Cuoghi A, Tomasi A, Guerzoni S, Ciccarese M, Pini LA. Validation of potential candidate biomarkers of drug-induced nephrotoxicity and allodynia in medication-overuse headache. J Headache Pain. 2015;16:559
– Schlatzer D, Maahs DM, Chance MR, Dazard JE, Li X, Hazlett F, Rewers M, Snell-Bergeon JK. Novel urinary protein biomarkers predicting the development of microalbuminuria and renal function decline in type 1 diabetes. Diabetes Care. 2012 Mar;35 (3):549-55
– Smith EM, Jorgensen AL, Midgley A, Oni L, Goilav B, Putterman C, Wahezi D, Rubinstein T, Ekdawy D, Corkhill R, Jones CA, Marks SD, Newland P, Pilkington C, Tullus K, Beresford MW. International validation of a urinary biomarker panel for identification of active lupus nephritis in children. Pediatr Nephrol. 2016 Sep 3;

