PEDF Human ELISA
PEDF is syntetized and released by human fetal retinal pigment epithelial cells (RPE) into the interphotoreceptor matrix and is localized to human chromosome 17p. It is a 50 kDa multifunctional glycoprotein belonging to the serpin protease inhibitor supergene (serpin) family, acting like substrates rather than inhibitors of serine proteases, being also described as serine peptidase inhibitor, clade F (alfa-2 antiplasmin, pigment epithelium derived factor), member 1. This gene encodes a 418 amino-acid protein with an asparagine glycosylation site at position 285–287 (Asn-Leu-Thr) and N-terminal signal peptide associated with secreted proteins. PEDF has an asymmetrical charge distribution, with a high density of basic residues concentrated on one side (positive) of the molecule and of acidic residues on the opposite side. Interactions of PEDF with three different types of molecules have been discovered: glycosaminoglycans of extracellular matrixes, collagens and receptors on the surface of neuronal cells. Negatively charged, acidic PEDF binds to collagen, lacks neurotrophic activity, and may confer antiangiogenic properties. PEDF has gliastatic, neuronotrophic, neuroprotective and antitumorigenic properties. PEDF acts in neuronal differentiation and survival in cells derived from retina and the central nervous system (CNS).Two functional epitopes have been identified on PEDF, a 34-mer peptide (residues 24–57) and a 44-mer peptide (residues 58–101). 44-mer peptide interacts with a a putative 80 kDa receptor (PEDFRN), identified on Y-79 cells (retinoblastoma cells), cerebellar and motor neurons, and in neural retina and replicates the neurotrophic function and the ability to block vascular leackage. The 34-mer peptide, possibly via a distinct receptor (PEDF-RA) identified on endothelial cells, induces apoptosis, blocks endothelial cell migration and corneal angiogenesis, but fails to induce Y-79 differentiation. Recently, PEDF was shown also to have potent anti-angiogenic activity as it specifically inhibited the migration of endothelial cells, an essential step in angiogenesis. Its activity equals or supersedes that of other anti-angiogenic factors, including angiostatin, endostatin and thrombospondin-1. In cell culture and in animal models, PEDF inhibited endothelial cell (EC) growth and migration and suppressed ischemia-induced neovascularization, whereas in porcine liver, the expression of PEDF has been associated with body muscularity and obesity. Analyses revealed that Human PEDF is correlated with BMI, CRP, diastolic blood pressure, insulin, Quicki. Individuals with metabolic syndrome (NCEP criterion) have significantly higher PEDF values than healthy subjects , suggesting that PEDF is and independent marker of MS with sufficient diagnostic efficacy.
The RD191114200R Human PEDF ELISA is a sandwich enzyme immunoassay for the quantitative measurement of human pigment epithelium-derived factor glycoprotein (PEDF).
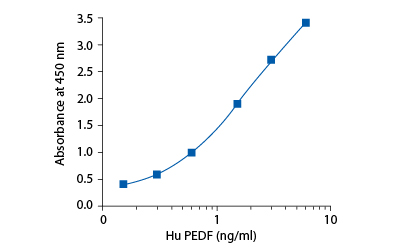
In the BioVendor Human PEDF ELISA, standards, quality controls and samples are incubated in microtitration wells coated with polyclonal anti-human PEDF antibody. After 60 minutes incubation and washing, biotin labelled polyclonal anti-human PEDF antibody is added and incubated with captured PEDF for 60 minutes. After another washing, streptavidin-HRP conjugate is added. After 60 minutes incubation and the last washing step, the remaining conjugate is allowed to react with the substrate solution (TMB). The reaction is stopped by addition of acidic solution and absorbance of the resulting yellow product is measured. The absorbance is proportional to the concentration of PEDF. A standard curve is constructed by plotting absorbance values against concentrations of standards, and concentrations of unknown samples are determined using this standard curve.
Intended use
Clinical Application
Test principle
Summary of protocol
– Catalioto RM, Valenti C, Liverani L, Giuliani S, Maggi CA. Characterization of a novel proinflammatory effect mediated by BK and the kinin B(2) receptor in human preadipocytes. Biochem Pharmacol. 2013 Aug 15;86 (4):508-20
– Chen C, Tso AW, Law LS, Cheung BM, Ong KL, Wat NM, Janus ED, Xu A, Lam KS. Plasma level of pigment epithelium-derived factor is independently associated with the development of the metabolic syndrome in Chinese men: a 10-year prospective study. J Clin Endocrinol Metab. 2010 Nov;95 (11):5074-81
– Famulla S, Lamers D, Hartwig S, Passlack W, Horrighs A, Cramer A, Lehr S, Sell H, Eckel J. Pigment epithelium-derived factor (PEDF) is one of the most abundant proteins secreted by human adipocytes and induces insulin resistance and inflammatory signaling in muscle and fat cells. Int J Obes (Lond). 2011 Jun;35 (6):762-72
– Hui E, Yeung CY, Lee PC, Woo YC, Fong CH, Chow WS, Xu A, Lam KS. Elevated circulating pigment epithelium-derived factor predicts the progression of diabetic nephropathy in patients with type 2 diabetes. J Clin Endocrinol Metab. 2014 Nov;99 (11):E2169-77
– Kanemura H, Go MJ, Nishishita N, Sakai N, Kamao H, Sato Y, Takahashi M, Kawamata S. Pigment epithelium-derived factor secreted from retinal pigment epithelium facilitates apoptotic cell death of iPSC. Sci Rep. 2013;3:2334
– Loegl J, Nussbaumer E, Hiden U, Majali-Martinez A, Ghaffari-Tabrizi-Wizy N, Cvitic S, Lang I, Desoye G, Huppertz B. Pigment epithelium-derived factor (PEDF): a novel trophoblast-derived factor limiting feto-placental angiogenesis in late pregnancy. Angiogenesis. 2016 Jun 8;
– Loegl J, Nussbaumer E, Cvitic S, Huppertz B, Desoye G, Hiden U. GDM alters paracrine regulation of feto-placental angiogenesis via the trophoblast. Lab Invest. 2017 Apr;97(4):409-418. doi: 10.1038/labinvest.2016.149
– Sabry JH, El-Shaaer OS, Hammady AME, Fallah AAE. SERUM PIGMENT EPITHELIUM DERIVED FACTOR AS A NEW MARKER IN THE METABOLIC SYNDROME. International Journal of Recent Scientific Research. Vol.8, Issue,3, pp.15862-15866. 28th March,2017.http://dx.doi.org/10.24327/ijrsr.2017.0803.0021
– Sorkio A, Haimi S, Verdoold V, Juuti-Uusitalo K, Grijpma D, Skottman H. Poly(trimethylene carbonate) as an elastic biodegradable film for human embryonic stem cell-derived retinal pigment epithelial cells. J Tissue Eng Regen Med. 2017 Jan 4. doi: 10.1002/term.2221
– Stejskal D, Karpisek M, Svestak M, Hejduk P, Sporova L, Kotolova H. Pigment epithelium-derived factor as a new marker of metabolic syndrome in Caucasian population. J Clin Lab Anal. 2010;24 (1):17-9
– Suzuki M, Tsujikawa M, Itabe H, Du ZJ, Xie P, Matsumura N, Fu X, Zhang R, Sonoda KH, Egashira K, Hazen SL, Kamei M. Chronic photo-oxidative stress and subsequent MCP-1 activation as causative factors for age-related macular degeneration. J Cell Sci. 2012 May 15;125 (Pt 10):2407-15
– Tschoner A, Sturm A, Ress C, Engl J, Kaser S, Laimer M, Laimer E, Klaus A, Tilg H, Patsch JR, Ebenbichler ChF. Effect of weight loss on serum pigment epithelium-derived factor levels. ECI. 2011 Jan;:6
– Yilmaz Y, Eren F, Ayyildiz T, Colak Y, Kurt R, Senates E, Tuncer I, Dolar E, Imeryuz N. Serum pigment epithelium-derived factor levels are increased in patients with biopsy-proven nonalcoholic fatty liver disease and independently associated with liver steatosis. Clin Chim Acta. 2011 Nov 20;412 (23-24):2296-9

