Leptin Mouse/Rat ELISA
Leptin is a protein hormone with important effects in metabolism and regulating body weight. It is a single-chain 16 kDa protein consisting of 146 amino acid residues and encoded by the obese (ob) gene. Leptin is expressed predominantly by adipocytes, small amounts of leptin are also secreted by cells in the epithelium of stomach and in the placenta. Leptin´s effect on body weight is mediated through effects on hypothalamic centers, where leptin receptors are highly expressed. Leptin has a dual action, it decreases the appetite and increases energy consumption.
A mutations in the ob gene of leptin or in the gene of leptin receptor causes hyperphagia, reduced energy expenditure, and severe obesity in the ob/ob mice. Ob gene knockout mice are also characterized by several metabolic abnormalities including hyperglucocorticoidemia, hyperglycaemia, hyperinsulinemia and insulin resistance. When ob/ob mice are treated with injections of leptin, they lose their excess fat and return to normal body weight. Studies have shown that leptin appears to be a significant regulator of reproductive function. In addition, leptin is involved in bone metabolism and plays a significant role as an immunomodulator.
Features
- The total assay time is less than four hours.
- The kit measures total serum leptin.
- Quality controls are mouse and rat serum based. No human sera are used.
Research topic
Animal studies, Diabetology – Other Relevant Products, Energy metabolism and body weight regulation, Reproduction
Type
Sandwich ELISA, Biotin-labelled antibody
Applications
Serum, Plasma-EDTA, Plasma-Heparin, Plasma-Citrate
Sample Requirements
7 µl/well
Storage/Expiration
Store the kit at 2–8°C. Under these conditions, the kit is stable until the expiration date (see label on the box).
Calibration Curve
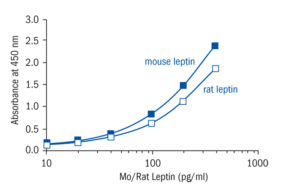
Calibration Range
100–4000 pg/ml
Limit of Detection
Analytical Limit of Detection is calculated from the real leptin values in wells and is 30 pg/ml for mouse leptin and 50 pg/ml for rat leptin
Intra-assay (Within-Run)
n = 8; CV = 2.2%
Inter-assay (Run-to-Run)
n = 8; CV = 3.4%
Spiking Recovery
94,50%
Dilutation Linearity
96,70%
Crossreactivity
bovine Non-detectable cat Non-detectable dog Non-detectable goat Non-detectable hamster Non-detectable horse Non-detectable monkey Non-detectable pig Non-detectable rabbit Non-detectable sheep Not tested chicken Not tested mouse Yes rat Yes human Yes (recommended dilution 1:3)
– Arimochi H, Sasaki Y, Kitamura A, Yasutomo K. Differentiation of preadipocytes and mature adipocytes requires PSMB8. Sci Rep. 2016;6:26791
– Bartels ED, Nielsen JM, Hellgren LI, Ploug T, Nielsen LB. Cardiac expression of microsomal triglyceride transfer protein is increased in obesity and serves to attenuate cardiac triglyceride accumulation. PLoS One. 2009;4 (4):e5300
– Bol VV, Delattre AI, Reusens B, Raes M, Remacle C. Forced catch-up growth after fetal protein restriction alters the adipose tissue gene expression program leading to obesity in adult mice. Am J Physiol Regul Integr Comp. 2009 Aug;297 (2):R291-9
– Bolze F, Bast A, Mocek S, Morath V, Yuan D, Rink N, Schlapschy M, Zimmermann A, Heikenwalder M, Skerra A, Klingenspor M. Treatment of diet-induced lipodystrophic C57BL/6J mice with long-acting PASylated leptin normalises insulin sensitivity and hepatic steatosis by promoting lipid utilisation. Diabetologia. 2016 Jun 7;
– De Jong A, Carreira S, Na N, Carillion A, Jiang C, Beuvin M, Lacorte JM, Bonnefont-Rousselot D, Riou B, Coirault C. Diaphragmatic function is enhanced in fatty and diabetic fatty rats. PLoS One. 2017 Mar 22;12(3):e0174043. doi:10.1371/journal.pone.0174043
– Giri S, Rattan R, Hag E, Khan M, Yasmin R, Won JS, Key L, Singh AK, Singh I. AICAR inhibits adipocyte differentiation in 3T3L1 and restores metabolic alterations in diet-induced obesity mice model. Nutr Metab (Lond) . Aug 10;3:31 (2006)
– Gout J, Sarafian D, Tirard J, Blondet A, Vigier M, Rajas F, Mithieux G, Begeot M, Naville D. Leptin infusion and obesity in mouse cause alterations in the hypothalamic melanocortin system. Obesity (Silver Spring). 2008 Aug;16 (8):1763-9
– Kenny S, Gamble J, Lyons S, Vlatkovic N, Dimaline R, Varro A, Dockray GJ. Gastric expression of plasminogen activator inhibitor (PAI)-1 is associated with hyperphagia and obesity in mice. Endocrinology. 2013 Feb;154 (2):718-26
– Kirk SL, Samuelsson AM, Argenton M, Dhonye H, Kalamatianos T, Poston L, Taylor PD, Coen CW. Maternal obesity induced by diet in rats permanently influences central processes regulating food intake in offspring. PLoS One. 2009;4 (6):e5870
– Putakala M, Gujjala S, Nukala S, Desireddy S. Beneficial Effects of Phyllanthus amarus Against High Fructose Diet Induced Insulin Resistance and Hepatic Oxidative Stress in Male Wistar Rats. Appl Biochem Biotechnol. 2017 Mar 28. doi: 10.1007/s12010-017-2461-0
– Ramirez-Lopez MT, Arco R, Decara J, Vazquez M, Noemi Blanco R, Alen F, Suarez J, Gomez de Heras R, Rodriguez de Fonseca F. Exposure to a Highly Caloric Palatable Diet during the Perinatal Period Affects the Expression of the Endogenous Cannabinoid System in the Brain, Liver and Adipose Tissue of Adult Rat Offspring. PLoS One. 2016;11 (11):e0165432
– Rodriguez L, Panadero MI, Rodrigo S, Roglans N, Otero P, Alvarez-Millan JJ, Laguna JC, Bocos C. Liquid fructose in pregnancy exacerbates fructose-induced dyslipidemia in adult female offspring. J Nutr Biochem. 2016 Jun;32:115-22
– Rohrbach S, Aurich AC, Li L, Niemann B. Age-associated loss in adiponectin-activation by caloric restriction: lack of compensation by enhanced inducibility of adiponectin paralogs CTRP2 and CTRP7. Mol Cell Endocrinol. 2007 Oct 15;277 (1-2):26-34
– Stejskal D, Karpisek M, Hanulova Z, Svestak M. Chemerin is an independent marker of the metabolic syndrome in a Caucasian population–a pilot study. Biomed Pap Med Fac Univ Palack. 2008 Dec;152 (2):217-21
– Tekin S, Erden Y, Ozyalin F, Cigremis Y, Colak C, Sandal S. The effects of intracerebroventricular infusion of irisin on feeding behaviour in rats. Neurosci Lett. 2017 Apr 3;645:25-32. doi: 10.1016/j.neulet.2017.02.066
– Tinkov AA, Gatiatulina ER, Popova EV, Polyakova VS, Skalnaya AA, Agletdinov EF, Nikonorov AA, Skalny AV. Early High-Fat Feeding Induces Alteration of Trace Element Content in Tissues of Juvenile Male Wistar Rats. Biol Trace Elem Res. 2016 Jun 16;
– Tolman JR, Lephart ED, Setchell KD, Eggett DL, Christensen MJ. Timing of supplementation of selenium and isoflavones determines prostate cancer risk factor reduction in rats. Nutr Metab (Lond). 2008;5:31
– Belemets N, Kobyliak N, Virchenko O, Falalyeyeva T, Olena T, Bodnar P, Savchuk O, Galenova T, Caprnda M, Rodrigo L, Skladany L, Delev D, Opatrilova R, Kruzliak P, Beregova T, Ostapchenko L. Effects of polyphenol compounds melanin on NAFLD/NASH prevention. Biomed Pharmacother. 2017 Apr;88:267-276. doi: 10.1016/j.biopha.2017.01.028

