IP-10 Human ELISA
IP-10 (Interferon-gamma inducible Protein 10kDa) also known as CXCL10, is secreted by several cell types in response to IFN-gamma and LPS. These cell types include monocytes, endothelial cells and fibroblasts [1]. The gene for IP-10 is located on chromosome 4 in a cluster among several other cytokines and encodes a 98 amino acid precursor protein [1].
IP-10 has been attributed to several roles, such as chemoattraction for monocytes and T cells (but not for neutrophils), inhibition of bone marrow colony formation and angiogenesis, promotion of T cells adhesion molecule expression [2, 3]. IP-10 shares a common receptor, CXCR3, with the chemokine MIG, but has been shown to play a distinct role in host defense in infections [4].
IP-10 expression has been associated with HIV infection [5], is involved in inflammatory skin disease [6] and other allergic diseases; it appears in inflammation of the nervous system and in Alzheimer’s disease (astrocytes expressing IP-10 are commonly associated with senile plaques) [7].
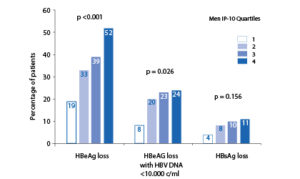
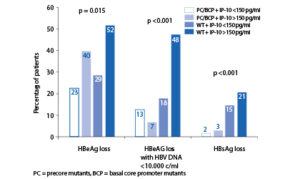
Pre-treatment IP-10 levels appear to predict response to peginterferon (PegIFN) therapy in chronic hepatitis C patients [8–11], independent of other known predictors, such as viral load, HCV genotype and stage of liver disease [8,10,11]. Peginterferon is also a first-line treatment option for chronic hepatitis B patients. Higher pre-treatment IP-10 levels are associated with an increased probability of HBeAg loss after PegIFN therapy. A combination of high baseline IP-10 and absence of precore (PC) and basal core promoter (BCP) mutants identified patients with the highest probability of combined response (HBeAg loss with HBV DNA <10,000 c/ ml) and HBsAg loss [12].
The RGP019R Human IP-10 ELISA kit is a solid phase sandwich ELISA for the in-vitro qualitative and quantitative determination of IP-10 (Interferon-gamma inducible Protein 10kDa) also known as CXCL10 in supernatants, buffered solutions or serum and plasma samples. This assay will recognise both natural and recombinant human IP-10.
A capture antibody highly specific for IP-10 has been coated to the wells of the microtitre strip plate provided during manufacture. Binding of IP-10 in samples and known standards to the capture antibodies is completed and then any excess unbound analyte is removed. During the next incubation period the binding of the biotinylated anti-IP-10 secondary antibody to the analyte occurs. Any excess unbound secondary antibody is then removed. The HRP conjugate solution is then added to every well including the zero wells, following incubation excess conjugate is removed by careful washing. A chromogen substrate is added to the wells resulting in the progressive development of a blue coloured complex with the conjugate. The colour development is then stopped by the addition of acid turning the resultant final product yellow. The intensity of the produced coloured complex is directly proportional to the concentration of IP-10 present in the samples and standards. The absorbance of the colour complex is then measured and the generated OD values for each standard are plotted against expected concentration forming a standard curve. This standard curve can then be used to accurately determine the concentration of IP-10 in any sample tested.
Intended use
Clinical Application
Test principle

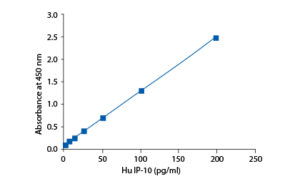
Precision
Intra-assay (Within-Run) (n=6)
Intra-assay (Within-Run) (n=18)
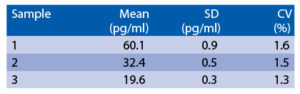
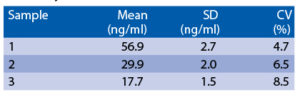
The spiking recovery was evaluated by spiking different concentrations of recombinant IP-10 in human serum. Recoveries ranged from 118.7% to 122.4%. The sensitivity, minimum detectable dose of IP-10 using this BioVendor IP-10 ELISA kit was found to be 5.7 pg/ml. This was determined by adding 3 standard deviations to the mean OD obtained when the zero standard was assayed 32 times.
Spiking recovery
Sensitivity
Summary of protocol
Baseline IP-10 and response at 6 months post-treatment. Relationship between baseline IP-10 and response to treatment in the HBeAg-positive population in the overall cohort. Observed response rates by baseline IP-10 and presence of PC and BCP mutants. The probability of combined response according to a baseline IP-10, in combination with the presence or absence of precore and/or core promoter mutants
Clinical Relevance
– Angiolillo AL, Sgadari C, Taub DD, Liao F, Farber JM, Maheshwari S, Kleinman HK, Reaman GH, Tosato G. Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. J Exp Med. 1995 Jul 1;182 (1):155-62
– Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol. 2002 Apr 1;168 (7):3195-204
– Luster AD, Unkeless JC, Ravetch JV. Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature. 1985 Jun 20-26;315 (6021):672-6
– Papatheodoridis G, Goulis J, Manolakopoulos S, Margariti A, Exarchos X, Kokkonis G, Hadziyiannis E, Papaioannou C, Manesis E, Pectasides D, Akriviadis E. Changes of HBsAg and interferon-inducible protein 10 serum levels in naive HBeAg-negative chronic hepatitis B patients under 4-year entecavir therapy. J Hepatol. 2013 Sep 5;
– Sebastiani S, Albanesi C, De PO, Puddu P, Cavani A, Girolomoni G. The role of chemokines in allergic contact dermatitis. Arch Dermatol Res. 2002 Jan;293 (11):552-9 – Strieter RM, Polverini PJ, Arenberg DA, Kunkel SL. The role of CXC chemokines as regulators of angiogenesis. Shock. 1995 Sep;4 (3):155-60

