Glucagon-Like Peptide-1 Human ELISA (Multispecies specificity)
GLP-1 is a peptide hormone from the intestinal mucosa, which is produced from its precursor, proglucagon by post transnational processing. The mammalian proglucagon 1) is synthesized in the neuroendocrine L-cell of the intestine and the alpha-cells of the pancreas. It contains within its structure the sequences of glucagon and two glucagon-like peptides (GLP-1 and GLP-2) in tandem flanked at their amino and carboxyl termini by dibasic residues. GLP-1 is a 37 amino acids peptide and produced in the small intestine and in the pancreas in the human, in either C-terminal-amidated on glycine-extended form.
GLP1 (7-36) amide and its receptor are present in several brain regions and may play a role in the physiologicalcontrol of feeding4). Several reports have been presented as follows as to the biological activities of GLP-1. GLP-1 (7-37) and (7-36) amide is known as one of the most potent insulin secretagogues. GLP-1 (7-36) amide was supposed to improved glycemic control in patients with type 2 diabetes by increasing insulin secretion, by inhibiting glucagon secretion and by delaying gastric emptying rather than by altering extrapancreatic glucose metabolism. Intravenous GLP-1(7-37) and (7-36)amide could normalize fasting hyperglycaemia in type 2 diabetic patients. Hyperglycaemia during parenteral nutrition could be controlled by exogenous GLP-1, whereas the chronic therapy of type 2 diabetes required GLP-1 derivatives with longer duration of action. Recombinant GLP-1 (7-36) amide was recently shown to cause significant weight loss in type 2 diabetics when administered for 6 weeks as a continuous subcutaneous infusion, 5-day treatment of hereby obese human subjects with GLP-1 at high doses by prandial subcutaneous infusion promptly slowed gastric emptying as a probable mechanism of action of increased satiety, decreased hunger and reduced food intake with an ensuing weight loss.
A G-protein-coupled receptor, GPR120, which is abundantly expressed in intestine, functions as a receptor for unsaturated long-chain FFAs (free fatty acids). The stimulation of GPR120 by FFAs promotes the secretion of GLP-1 in vitro (measured by YK160, Yanaihara Institute Inc) and in vivo, and increases circulation insulin, indicate that GPR120-mediated GLP-1 secretion induced by dietary FFAs is important in the treatment of diabetes.
All these approaches have shown remarkable efficacy in both experimental and clinical studies. The GLP-1-based therapy of type 2 diabetes, therefore, represents a new and attractive alternative. Yanaihara Institute Inc. developed a quantitative EIA kit with high specificity and sensitivity (detection limit 0.206 ng/mL) for rat/mouse/human GLP-1 as a useful tool for these necessaries.
Research topic
Animal studies, Diabetology – Other Relevant Products, Energy metabolism and body weight regulation
Type
Competitive ELISA, Immobilized antibody
Applications
Plasma
Storage/Expiration
Store the complete kit at 2–8°C. Under these conditions, the kit is stable until the expiration date (see label on the box).
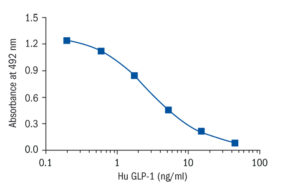
Calibration Curve
Note
For sample collection we recommend using vacutainers containing EDTA and DPP4 inhibitor.
– Aronis KN, Chamberland JP, Mantzoros CS. GLP-1 promotes angiogenesis in human endothelial cells in a dose-dependent manner, through the Akt, Src and PKC pathways. Metabolism. 2013 Sep;62 (9):1279-86
– Rauchenzauner M, Laimer M, Wiedmann M, Tschoner A, Salzmann K, Sturm W, Sandhofer A, Walser G, Luef G, Ebenbichler CF. The novel insulin resistance parameters RBP4 and GLP-1 in patients treated with valproic acid: just a sidestep?. Epilepsy Res. 2013 May;104 (3):285-8
– Salis ER, Reith DM, Wheeler BJ, Broadbent RS, Medlicott NJ. Insulin resistance, glucagon-like peptide-1 and factors influencing glucose homeostasis in neonates. Arch Dis Child Fetal Neonatal . 2016 Sep 2;
– Zahradka P, Wright B, Weighell W, Blewett H, Baldwin A, O K, Guzman RP, Taylor CG. Daily non-soy legume consumption reverses vascular impairment due to peripheral artery disease. Atherosclerosis. 2013 Oct;230 (2):310-4

