Fibroblast Growth Factor 21 Mouse/Rat ELISA
The FGFs are a family of more than 20 small (~17–26 kDa) secreted peptides. The initial characterization of these proteins focused on their ability to stimulate fibroblast proliferation. This mitogenic activity was mediated through FGF receptors (FGFRs) 1, 2, or 3. A fourth closely related tyrosine kinase receptor (FGFR4) was able to bind the FGFs but did not lead to a mitogenic response.
FGFs modulate cellular activity via at least 5 distinct subfamilies of high-affinity FGF receptors (FGFRs): FGFR-1, –2, –3, and –4, all with intrinsic tyrosine kinase activity and, except for FGFR-4, multiple splice isoforms, and FGFR-5, which lacks an intracellular kinase domain. There is growing evidence that FGFRs can be important for regulation of glucose and lipid homeostasis. The overexpression of a dominant negative form of FGFR-1 in β cells leads to diabetes in mice, which thus implies that proper FGF signaling is required for normal β cell function and glycemia maintenance. FGFR-2 appears to be a key molecule during pancreatic development. Moreover, FGFR-4 has been implicated in cholesterol metabolism and bile acid synthesis.
FGF-19, has been shown to cause resistance to diet-induced obesity and insulin desensitization and to improve insulin, glucose, and lipid profiles in diabetic rodents. Since these effects, at least in part, are mediated through the observed changes in metabolic rates, FGF-19 can be considered as a regulator of energy expenditure.
FGF-21 is preferentially expressed in liver, but an exact knowledge of FGF-21 bioactivity and its mode of action have been lacking to date. FGF-21 is a potent activator of glucose uptake on adipocytes, protects animals from diet-induced obesity when overexpressed in transgenic mice, and lowers blood glucose and triglyceride levels when therapeutically administered to diabetic rodents.
Research topic
Animal studies, Diabetology – Other Relevant Products, Energy metabolism and body weight regulation
Type
Sandwich ELISA, Biotin-labelled antibody
Applications
Serum
Sample Requirements
mouse samples: 7 µl/well
rat samples: 50 µl/well
Storage/Expiration
Store the complete kit at 2–8°C. Under these conditions, the kit is stable until the expiration date (see label on the box).
Calibration Curve
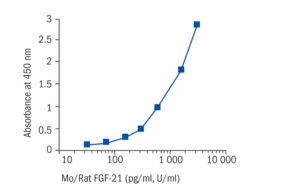
Calibration Range
40–2560 pg/ml
Limit of Detection
18.4 pg/ml
Intra-assay (Within-Run)
mouse samples CV = 8.4%
rat samples CV = 8.5%
Inter-assay (Run-to-Run)
mouse samples CV = 8.7%
rat samples CV = 6%
Spiking Recovery
mouse samples 98.9%
rat samples 104.5
Dilutation Linearity
mouse samples 99.8%
rat samples 103.7%
Crossreactivity
| bovine | Weak reactivity (recom. dilution 1:3) |
|---|---|
| cat | Yes (recommended dilution 1:3) |
| dog | Non-detectable |
| horse | Non-detectable |
| goat | Yes (recommended dilution 1:6) |
| hamster | Yes (recommended dilution 1:12) |
| human | Non-detectable |
| monkey | Non-detectable |
| rabbit | Non-detectable |
| chicken | Not tested |
| mouse | Yes |
| rat | Yes |
| sheep | Yes (recommended dilution 1:3) |
| pig | Yes (recommended dilution 1:3) |
– Bookout AL, de Groot MH, Owen BM, Lee S, Gautron L, Lawrence HL, Ding X, Elmquist JK, Takahashi JS, Mangelsdorf DJ, Kliewer SA. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat Med. 2013 Sep;19 (9):1147-52
– Bornstein S, Brown SA, Le PT, Wang X, DeMambro V, Horowitz MC, MacDougald O, Baron R, Lotinun S, Karsenty G, Wei W, Ferron M, Kovacs CS, Clemmons D, Wan Y, Rosen CJ. FGF-21 and skeletal remodeling during and after lactation in C57BL/6J mice. Endocrinology. 2014 Sep;155 (9):3516-26
– Chun S, Bamba T, Suyama T, Ishijima T, Fukusaki E, Abe K, Nakai Y. A High Phosphorus Diet Affects Lipid Metabolism in Rat Liver: A DNA Microarray Analysis. PLoS One. 2016;11 (5):e0155386
– Cornu M, Oppliger W, Albert V, Robitaille AM, Trapani F, Quagliata L, Fuhrer T, Sauer U, Terracciano L, Hall MN. Hepatic mTORC1 controls locomotor activity, body temperature, and lipid metabolism through FGF21. Proc Natl Acad Sci U S A. 2014 Aug 12;111 (32):11592-9
– Cui Y, Giesy SL, Hassan M, Davis K, Zhao S, Boisclair YR. Hepatic FGF21 production is increased in late pregnancy in the mouse. Am J Physiol Regul Integr Comp. 2014 Aug 1;307 (3):R290-8
– Feingold KR, Grunfeld C, Heuer JG, Gupta A, Cramer M, Zhang T, Shigenaga JK, Patzek SM, Chan ZW, Moser A, Bina H, Kharitonenkov A. FGF21 Is Increased by Inflammatory Stimuli and Protects Leptin-Deficient ob/ob Mice from the Toxicity of Sepsis. Endocrinology. 2012 Jun;153 (6):2689-700
– Fisher FM, Chui PC, Antonellis PJ, Bina HA, Kharitonenkov A, Flier JS, Maratos-Flier E. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes. 2010 Nov;59 (11):2781-9
– Honda Y, Kessoku T, Ogawa Y, Tomeno W, Imajo K, Fujita K, Yoneda M, Takizawa T, Saito S, Nagashima Y, Nakajima A. Pemafibrate, a novel selective peroxisome proliferator-activated receptor alpha modulator, improves the pathogenesis in a rodent model of nonalcoholic steatohepatitis. Sci Rep. 2017 Feb 14;7:42477. doi: 10.1038/srep42477
– Jornayvaz FR, Jurczak MJ, Lee HY, Birkenfeld AL, Frederick DW, Zhang D, Zhang XM, Samuel VT, Shulman GI. A high-fat, ketogenic diet causes hepatic insulin resistance in mice, despite increasing energy expenditure and preventing weight gain. Am J Physiol Endocrinol Metab. 2010 Nov;299 (5):E808-15
– Laeger T, Albarado DC, Burke SJ, Trosclair L, Hedgepeth JW, Berthoud HR, Gettys TW, Collier JJ, Munzberg H, Morrison CD. Metabolic Responses to Dietary Protein Restriction Require an Increase in FGF21 that Is Delayed by the Absence of GCN2. Cell Rep. 2016 Jul 6;
– Laeger T, Henagan TM, Albarado DC, Redman LM, Bray GA, Noland RC, Munzberg H, Hutson SM, Gettys TW, Schwartz MW, Morrison CD. FGF21 is an endocrine signal of protein restriction. J Clin Invest. 2014 Sep 2;124 (9):3913-22
– Osorio JS, Trevisi E, Ballou MA, Bertoni G, Drackley JK, Loor JJ. Effect of the level of maternal energy intake prepartum on immunometabolic markers, polymorphonuclear leukocyte function, and neutrophil gene network expression in neonatal Holstein heifer calves. J Dairy Sci. 2013 Jun;96 (6):3573-87
– Owen BM, Bookout AL, Ding X, Lin VY, Atkin SD, Gautron L, Kliewer SA, Mangelsdorf DJ. FGF21 contributes to neuroendocrine control of female reproduction. Nat Med. 2013 Sep;19 (9):1153-6
– Patel V, Adya R, Chen J, Ramanjaneya M, Bari MF, Bhudia SK, Hillhouse EW, Tan BK, Randeva HS. Novel insights into the cardio-protective effects of FGF21 in lean and obese rat hearts. PLoS One. 2014;9 (2):e87102
– Quesada-Lopez T, Cereijo R, Turatsinze JV, Planavila A, Cairo M, Gavalda-Navarro A, Peyrou M, Moure R, Iglesias R, Giralt M, Eizirik DL, Villarroya F. The lipid sensor GPR120 promotes brown fat activation and FGF21 release from adipocytes. Nat Commun. 2016 Nov 17;7:13479
– Vandanmagsar et al. Impaired Mitochondrial Fat Oxidation Induces FGF21 in Muscle. Cell Reports. May 24, 2016;15:1–14
– Villarroya J, Flachs P, Redondo-Angulo I, Giralt M, Medrikova D, Villarroya F, Kopecky J, Planavila A. Fibroblast Growth Factor-21 and the Beneficial Effects of Long-Chain n-3 Polyunsaturated Fatty Acids. Lipids. 2014 Sep 10;
– von Holstein-Rathlou S, BonDurant LD, Peltekian L, Naber MC, Yin TC, Claflin KE, Urizar AI, Madsen AN, Ratner C, Holst B, Karstoft K, Vandenbeuch A, Anderson CB, Cassell MD, Thompson AP, Solomon TP, Rahmouni K, Kinnamon SC, Pieper AA, Gillum MP, Potthoff MJ. FGF21 Mediates Endocrine Control of Simple Sugar Intake and Sweet Taste Preference by the Liver. Cell Metab. 2016 Feb 9;23 (2):335-43
– Chen X, Ward SC, Cederbaum AI, Xiong H, Lu Y. Alcoholic fatty liver is enhanced in CYP2A5 knockout mice: The role of the PPARα-FGF21 axis. Toxicology. 2017 Mar 15;379:12-21. doi: 10.1016/j.tox.2017.01.016
– Zhou M, Luo J, Chen M, Yang H, Learned RM, DePaoli AM, Tian H, Ling L. Mouse species-specific control of hepatocarcinogenesis and metabolism by FGF19/FGF15. J Hepatol. 2017 Feb 9. pii: S0168-8278(17)30062-4. doi: 10.1016/j.jhep.2017.01.027

