Fibroblast Growth Factor 19 Human ELISA
Fibroblast growth factors (FGFs) are a large family of small (17-26 kDa) polypeptide growth factors found in organisms ranging from nematodes to humans. The FGF family has at least 22 members in vertebrates and share 13-71% amino acid identity. The initial characterization of these proteins focused on their ability to stimulate fibroblast proliferation. During embryonic development, FGFs have diverse roles in regulating cell proliferation, migration and differentiation. In the adult organism, FGFs are homeostatic factors and function in tissue repair and response to injury.
FGF signaling is mediated through one of four FGF receptors (FGFR1-FGFR4), a complex family of transmembrane receptor tyrosine kinases. FGFR5 has also been described, but lacks the kinase domain and signaling capability. FGFs have a high affinity for heparin sulfate proteoglycans and require heparin sulfate to activate FGF receptors. Although multiple FGFs interact with each of the four FGFRs, a novel fibroblast growth factor FGF-19 exhibits exclusive binding to only one of FGF receptors (FGFR4).
The normal function of FGF-19 has not been resolved, although its role in inner ear development has been suggested. It has been also found that hepatocyte expression of FGF-19 is induced by the transcription factor, farnesoid X receptor (FXR). FXR is a key regulator of cholesterol metabolism through suppression of the catabolic enzyme cyp7a, the first and rate-limiting step in the biosynthesis of bile acids (BA). A recent study found that, in humans, circulating FGF-19 has a diurnal rhythm controlled by the transintestinal BA flux, and that FGF-19 modulates hepatic BA synthesis. Through its systemic effects, circulating FGF-19 may also mediate other known BA-dependent effects on lipid and carbohydrate metabolism.
In transgenic mice expressing human FGF-19, researchers found a significant increased metabolic rate as well as decreased body weight and adiposity. Additionally, resistance to both diet-induced obesity and insulin desensitization were found. Similar responses have been observed when recombinant FGF-19 was injected into the mice. However, it has been shown too, that FGF-19 transgenic mice develop hepatic adrenocarcinomas with age, and recombinant FGF-19 treated mice exhibit proliferation of hepatocytes.
Areas of investigation:
Cholesterol metabolism, Metabolic syndrome
Type
Sandwich ELISA, Biotin-labelled antibody
Applications
Serum, Plasma-EDTA, Plasma-Heparin, Plasma-Citrate
Sample Requirements
50 µl/well
Storage/Expiration
Store the complete kit at 2–8°C. Under these conditions, the kit is stable until the expiration date (see
label
on the box).
Calibration Curve
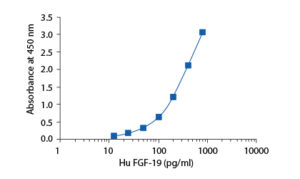
Calibration Range
12.5–800 pg/ml
Limit of Detection
4.8 pg/ml
Intra-assay (Within-Run)
n = 8; CV = 6.0%
Inter-assay (Run-to-Run)
n = 6; CV = 7.5%
Spiking Recovery
90,60%
Dilutation Linearity
96,30%
Crossreactivity
bovine Non-detectable
cat Not tested
goat Non-detectable
hamster Non-detectable
dog Not tested
horse Yes (recommended dilution 1:3)
mouse Non-detectable
rabbit Non-detectable
rat Non-detectable
sheep Non-detectable
pig Not tested
chicken Not tested
human Yes
monkey Yes (recommended dilution 1:3)
– Buzga M, Evzen M, Pavel K, Tomas K, Vladislava Z, Pavel Z, Svagera Z. Effects of the Intragastric Balloon MedSil(R) on Weight Loss, Fat Tissue, Lipid Metabolism, and Hormones Involved in Energy Balance. Obes Surg. 2014 Feb 1;
– Dostalova I, Kavalkova P, Haluzikova D, Lacinova Z, Mraz M, Papezova H, Haluzik M. Plasma concentrations of fibroblast growth factors 19 and 21 in patients with anorexia nervosa. J Clin Endocrinol Metab. 2008 Jun 17;
– Eren F, Kurt R, Ermis F, Atug O, Imeryuz N, Yilmaz Y. Preliminary evidence of a reduced serum level of fibroblast growth factor 19 in patients with biopsy-proven nonalcoholic fatty liver disease. Clinical Biochemistry. 2012;
– Gallego-Escuredo JM, Gomez-Ambrosi J, Catalan V, Domingo P, Giralt M, Fruhbeck G, Villarroya F. Opposite alterations in FGF21 and FGF19 levels and disturbed expression of the receptor machinery for endocrine FGFs in obese patients. Int J Obes (Lond). 2014 May 12;
– Li Z, Lin B, Lin G, Wu Y, Jie Y, Li X, Ko B, Chong Y, Luo J. Circulating FGF19 closely correlates with bile acid synthesis and cholestasis in patients with primary biliary cirrhosis. PLoS One. 2017 Jun 1;12(6):e0178580. doi: 10.1371/journal.pone.0178580. eCollection 2017. PubMed PMID: 28570655.
– Nielsen S, Svane MS, Kuhre RE, Clausen TR, Kristiansen VB, Rehfeld JF, Holst JJ, Madsbad S, Bojsen-Moller KN. Chenodeoxycholic acid stimulates glucagon-like peptide-1 secretion in patients after Roux-en-Y gastric bypass. Physiol Rep. 2017 Feb;5(3). pii: e13140. doi: 10.14814/phy2.13140
– Zhou M, Luo J, Chen M, Yang H, Learned RM, DePaoli AM, Tian H, Ling L. Mouse species-specific control of hepatocarcinogenesis and metabolism by FGF19/FGF15. J Hepatol. 2017 Feb 9. pii: S0168-8278(17)30062-4. doi: 10.1016/j.jhep.2017.01.027
– Zöhrer E, Anna A, Jahnel J, Moscla A, Della Corte C, Crudele A, Fauler G, Nobili V. Efficacy of docosahexaenoic acid-choline-vitamin E (DHA-CHO-VE) in paediatric NASH: a randomized controlled clinical trial. Appl Physiol Nutr Metab. 2017 May 16. doi: 10.1139/apnm-2016-0689

