Cystatin C Assay
Cystatin C Assay is an in vitro diagnostic test for the quantitative determination of Cystatin C in serum or plasma.
Cystatin C Assay is a convenient cost effective dual liquid stable latex enhanced immunoturbidimetric method. This critically important emerging marker aids in the early detection and diagnosis of renal disease. The assay is highly sensitive with a sensitivity of 0.068 mg/dL, and an extended linear range from 2.0 – 8.0 mg/dL. The method has an excellent correlation with existing commercially available products and is highly precise with intra and inter-assay precision CV% of < 5.0%. Packaged for optimal operator convenience, reagent transfer can be eliminated for most chemistry systems with instrument specific packaging options including Roche™ Hitachi 917 series, Beckman AU (400/600/640/680), Beckman Synchron CX, DXC and Dimension/Expand.
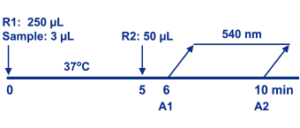
Cystatin C Assay is based on a latex enhanced immunoturbidimetric assay. Cystatin C in the sample binds to the specific anti-cystatin C antibody, which is coated on latex particles, and causes agglutination. The degree of the turbidity caused by agglutination can be measured optically and is proportional to the amount of cystatin C in the sample. The instrument calculates the cystatin C concentration of a patient specimen by interpolation of the obtained signal of a 6-point calibration curve.