Cartilage Oligomeric Matrix Protein Human, Mouse Monoclonal Antibody, Clone: 16F12
Cartilage oligomeric matrix protein (COMP), also designated thrombospondin 5 (TSP 5), is non-collagenous glycoprotein and is a member of the thrombospondin family of extracellular proteins. COMP is a calcium-binding protein of high molecular weight (>500kDa) present in the extracellular matrix of articular, nasal and tracheal cartilage. COMP is not only cartilage-derived but was found widely in other tissues, including synovium and tendon. Intact COMP is pentameric, with five identical subunits and the carboxy-terminal globular domain of native COMP binds to collagens I, II, and IX. It has been proposed that COMP molecules are important for maintaining the properties and integrity of collagen network. In addition COMP may have a storage and delivery function for hydrophobic cellsignaling molecules such as vitamin D. The significance of COMP for normal development and function of cartilage has been underscored by the discovery that mutations of the COMP gene result in pseudoachondroplasia and some forms of multiple epiphyseal dysplasia. Most published studies have shown that serum levels of COMP provide important information about metabolic changes occurring in the cartilage matrix in joint disease. These studies describe that serum COMP level correlated with cartilage degradation and is a potential prognostic marker in inflammatory joint diseases such as osteoarthritis (OA) and rheumatoid arthritis (RA). Results have demonstrated an association of increasing serum COMP levels with progressive destruction of articular cartilage monitored radiographically. OA and RA are a common disease causing pain and disability in a significant proportion of the adult population and early diagnostics of these diseases is very important for future therap
Type
Monoclonal Antibody
Applications
Western blotting, ELISA, Immunohistochemistry
Antibodies Applications
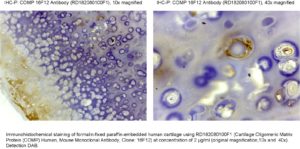
Source of Antigen
Human articular cartilage
Hosts
Mouse
Isotype
IgG1
Clone
16F12
Preparation
The antibody is a mouse monoclonal antibody against Human COMP.
Purification Method
Affinity chromatography on a column with immobilized protein G.
Antibody Content
0.1 mg (determined by BCA method)
Formulation
The antibody is lyophilized in 0.05 M phosphate buffer, 0.1 M NaCl, pH 7.2. AZIDE FREE.
Reconstitution
Add 0.1 ml of deionized water and let the lyophilized pellet dissolve completely. Slight turbidity may occur after reconstitution, which does not affect activity of the antibody. In this case clarify the solution by centrifugation.
Shipping
At ambient temperature. Upon receipt, store the product at the temperature recommended below.
Storage/Expiration
The lyophilized antibody remains stable and fully active until the expiry date when stored at –20°C. Aliquot the product after reconstitution to avoid repeated freezing/thawing cycles and store frozen at –80°C. Reconstituted antibody can be stored at 4°C for a limited period of time; it does not show decline in activity after one week at 4°C.
Quality Control Test
Indirect ELISA – to determine titer of the antibody
SDS PAGE – to determine purity of the antibody
Note
This product is for research use only.
– Jordan JM, Luta G, Stabler T, Renner JB, Dragomir AD, Vilim V, Hochberg MC, Helmick CG, Kraus VB. Ethnic and sex differences in serum levels of cartilage oligomeric matrix protein: the Johnston County Osteoarthritis Project. Arthritis Rheum. 2003 Mar;48 (3):675-81
– Shen CY, Wu CL, Lin YM, Hwang RC. Sensitive and Label-free Detection of Cartilage Oligomeric Matrix Protein on Quartz Crystal Microbalance. IJNTR. March 2016;2 (3):45-48
– Vilim V, Lenz ME, Vytasek R, Masuda K, Pavelka K, Kuettner KE, Thonar EJ. Characterization of monoclonal antibodies recognizing different fragments of cartilage oligomeric matrix protein in human body fluids. Arch Biochem Biophys. 1997 May 1;341 (1):8-16
– Vilim V, Voburka Z, Vytasek R, Senolt L, Tchetverikov I, Kraus VB, Pavelka K. Monoclonal antibodies to human cartilage oligomeric matrix protein: epitope mapping and characterization of sandwich ELISA. Clin Chim Acta. 2003 Feb;328 (1-2):59-69
– Vilim V, Vytasek R, Olejarova M, Machacek S, Gatterova J, Prochazka B, Kraus VB, Pavelka K. Serum cartilage oligomeric matrix protein reflects the presence of clinically diagnosed synovitis in patients with knee osteoarthritis. Osteoarthritis Cartilage. 2001 Oct;9 (7):612-8
– Wang SH, Shen CY, Weng TC, Lin PH, Yang JJ, Chen IF, Kuo SM, Chang SJ, Tu YK, Kao YH, Hung CH. Detection of cartilage oligomeric matrix protein using a quartz crystal microbalance. Sensors. 2010;10:11633-11634

