Cartilage Oligomeric Matrix Protein Human ELISA
Cartilage oligomeric matrix protein (COMP), also designated thrombospondin 5 (TSP 5), is non-collagenous glycoprotein and is a member of the thrombospondin family of extracellular proteins. COMP is a calcium-binding protein of high molecular weight (>500kDa) present in the extracellular matrix of articular, nasal and tracheal cartilage. COMP is not only cartilage-derived but was found widely in other tissues, including synovium and tendon. Intact COMP is pentameric, with five identical subunits and the carboxy-terminal globular domain of native COMP binds to collagens I, II, and IX. It has been proposed that COMP molecules are important for maintaining the properties and integrity of collagen network. In addition COMP may have a storage and delivery function for hydrophobic cellsignaling molecules such as vitamin D. The significance of COMP for normal development and function of cartilage has been underscored by the discovery that mutations of the COMP gene result in pseudoachondroplasia and some forms of multiple epiphyseal dysplasia. Most published studies have shown that serum levels of COMP provide important information about metabolic changes occurring in the cartilage matrix in joint disease. These studies describe that serum COMP level correlated with cartilage degradation and is a potential prognostic marker in inflammatory joint diseases such as osteoarthritis (OA) and rheumatoid arthritis (RA). Results have demonstrated an association of increasing serum COMP levels with progressive destruction of articular cartilage monitored radiographically. OA and RA are a common disease causing pain and disability in a significant proportion of the adult population and early diagnostics of these diseases is very important for future therapy.
Features
- European Union: for in vitro diagnostic use
- Rest of the world: for research use only!
- The total assay time is less than 3.5 hours
- The kit measures COMP in serum and plasma (EDTA, citrate, heparin)
- Assay format is 96 wells
- Quality Controls are human serum based
- Standard is recombinant protein based
- Components of the kit are provided ready to use, concentrated or lyophilized
Research topic
Bone and cartilage metabolism
Type
Sandwich ELISA, Biotin-labelled antibody
Applications
Serum, Plasma-EDTA, Plasma-Heparin, Plasma-Citrate
Sample Requirements
5 µl/well
Storage/Expiration
Store the kit at 2–8°C. Under these conditions, the kit is stable until the expiration date (see label on the box).
Calibration Curve
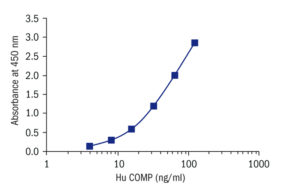
Calibration Range
4–128 ng/ml
Limit of Detection
0.4 ng/ml
Intra-assay (Within-Run)
n = 8; CV = 6.0%
Inter-assay (Run-to-Run)
n = 8; CV = 4.9%
Spiking Recovery
97,60%
Dilutation Linearity
95,80%
Crossreactivity
| bovine | Non-detectable |
|---|---|
| cat | Non-detectable |
| dog | Non-detectable |
| goat | Non-detectable |
| hamster | Non-detectable |
| horse | Non-detectable |
| monkey | Non-detectable |
| mouse | Non-detectable |
| pig | Non-detectable |
| rabbit | Non-detectable |
| rat | Non-detectable |
| sheep | Non-detectable |
| chicken | Not tested |
| human | Yes |
– Bruyere O, Collette JH, Ethgen O, Rovati LC, Giacovelli G, Henrotin YE, Seidel L, Reginster JY. Biochemical markers of bone and cartilage remodeling in prediction of longterm progression of knee osteoarthritis. J Rheumatol. 2003 May;30 (5):1043-50
– Chandran V, Cook RJ, Edwin J, Shen H, Pellett FJ, Shanmugarajah S, Rosen CF, Gladman DD. Soluble biomarkers differentiate patients with psoriatic arthritis from those with psoriasis without arthritis. Rheumatology (Oxford). 2010 Jul;49 (7):1399-405
– Coan HB, Curran JE, Dyer TD, Kent Jr. JW, Choudary A, Nicolella DP, Carless MA, Kumar S, Almeida MA, Duggirala R, Glahn DC, Mahaney MC, Blangero J, Havill LM. Variation in osteoarthritis biomarker serum comp levels in Mexican Americans is associated with SNPs in a region of chromosome 22q encompassing MICAL3, BCL2L13, and BID. Osteoarthritis and Cartilage. April 2013;21:S172
– Dragomir AD, Kraus VB, Renner JB, Luta G, Clark A, Vilim V, Hochberg MC, Helmick CG, Jordan JM. Serum cartilage oligomeric matrix protein and clinical signs and symptoms of potential pre-radiographic hip and knee pathology. Osteoarthritis Cartilage. 2002 Sep;10 (9):687-91
– Jordan JM, Luta G, Stabler T, Renner JB, Dragomir AD, Vilim V, Hochberg MC, Helmick CG, Kraus VB. Ethnic and sex differences in serum levels of cartilage oligomeric matrix protein: the Johnston County Osteoarthritis Project. Arthritis Rheum. 2003 Mar;48 (3):675-81
– Lattermann C, Jacobs CA, Bunnell MP, Jochimsen K, Abt J, Reinke EK, Gammon L, Huebner JL, Kraus VB, Spindler KP. Logistical challenges and design considerations for studies using acute anterior cruciate ligament injury as a potential model for early posttraumatic osteoarthritis. J Orthop Res. 2016 Jun 9;
– Misumi K, Vilim V, Clegg PD, Thompson CC, Carter SD. Measurement of cartilage oligomeric matrix protein (COMP) in normal and diseased equine synovial fluids. Osteoarthritis Cartilage. 2001 Feb;9 (2):119-27
– Stabler T, Fang F, Jordan J, Vilim V, Kraus VB. A comparison of methods for measuring cartilage oligomeric protein (COMP) in human subjects with knee OA.
– Stidham RW, Wu J, Shi J, Lubman DM, Higgins PD. Serum Glycoproteome Profiles for Distinguishing Intestinal Fibrosis from Inflammation in Crohn’s Disease. PLoS One. 2017 Jan 23;12(1):e0170506. doi: 10.1371/journal.pone.0170506
– Swärd P, Struglics A, Roos H, Englund M, Boegård T, Frobell R. Knee injury with concomitant osteochondral fracture is associated with increased synovial fluid concentrations of TNF-α. Osteoarthritis and Cartilage. April 2013;21:S7
– Vilim V, Lenz ME, Vytasek R, Masuda K, Pavelka K, Kuettner KE, Thonar EJ. Characterization of monoclonal antibodies recognizing different fragments of cartilage oligomeric matrix protein in human body fluids. Arch Biochem Biophys. 1997 May 1;341 (1):8-16
– Vilim V, Olejarova M, Machacek S, Gatterova J, Kraus VB, Pavelka K. Serum levels of cartilage oligomeric matrix protein (COMP) correlate with radiographic progression of knee osteoarthritis. Osteoarthritis Cartilage. 2002 Sep;10 (9):707-13
– Vilim V, Voburka Z, Vytasek R, Senolt L, Tchetverikov I, Kraus VB, Pavelka K. Monoclonal antibodies to human cartilage oligomeric matrix protein: epitope mapping and characterization of sandwich ELISA. Clin Chim Acta. 2003 Feb;328 (1-2):59-69
– Vilim V, Vytasek R, Olejarova M, Machacek S, Gatterova J, Prochazka B, Kraus VB, Pavelka K. Serum cartilage oligomeric matrix protein reflects the presence of clinically diagnosed synovitis in patients with knee osteoarthritis. Osteoarthritis Cartilage. 2001 Oct;9 (7):612-8
– Wang SH, Shen CY, Weng TC, Lin PH, Yang JJ, Chen IF, Kuo SM, Chang SJ, Tu YK, Kao YH, Hung CH. Detection of cartilage oligomeric matrix protein using a quartz crystal microbalance. 2010;10:11633-11634

