Arginase Liver Type Human ELISA
Arginase [EC 3.5.3.1; L-arginine aminohydrolase] is an enzyme that hydrolyzes Larginine to L-ornithine and urea in the urea cycle. Two forms of arginase exists which are designed as arginase I and arginase II. Liver-type arginase I is expressed primarily in the liver and to some extend in the erythrocytes. Arginase II is expressed in many extrahepatic tissues, such as brain, spinal cord, kidney, small intestine and mammary gland. Although arginase I and arginase II have similar enzyme activities, they have different pI, immunological reactivity and are encoded by different genes. Human arginase I is a 35 kDa protein circulating in blood probably as a homotrimer. Circulating liver-type arginase was clinically used as a liver specific marker which may reflect not only early occurrence of liver injury but also early termination of liver injury. The measurement of liver-type arginase is clinically applicable for monitoring conditions of patients with liver disorders or pre- and postoperative conditions of patients who received partial hepatectomy with quicker normalization in comparison with aminotransferases (ALT and AST). Recently, arginase I gene was found to be one of the most prominent among astma genes. In situ hybridization demonstrated marked staining of arginase I in submucosal inflammatory lesions and arginase activity increased in allergen challenged lungs. Finally, it was found that both arginase I was the most significantly up-regulated protein in the murine spinal cord during experimental autoimmune encephalomyelitis. The results indicated that arginase I played important roles in autoimmune inflammation in the central nervous system.
Features
- It is intended for research use only
- The total assay time is less than 3 hours
- The kit measures total Arginase I (Liver-Type) in serum and cerebrospinal fluid (CSF)
- Assay format is 96 wells
- Standard is recombinant protein based
- Serum samples require very careful preparation. The erythrocytes have to be spinned down immediately (within few seconds) after taking blood to avoid hemolysis and contamination of the sample with erythrocyte arginase
- Components of the kit are provided ready to use, concentrated or lyophilized
Research topic
Asthma and allergic rhinitis, Blood pressure regulation and NO metabolism, Immunology, Oncology, Pulmonary diseases
Type
Sandwich ELISA, HRP-labelled antibody
Applications
Serum, Cerebrospinal fluid
Sample Requirements
25 µl/well
Storage/Expiration
Place the lyophilized Master Standards and Quality Controls at –20 °C after the kit delivery. Store the other kit components at 2–8°C. Under these conditions the kit is stable till the expiry date is over. (See the expiry date indicated on the kit label).
Calibration Curve
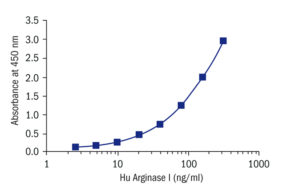
Calibration Range
5–320 ng/ml
Limit of Detection
0.5 ng/ml
Intra-assay (Within-Run)
n = 8; CV = 5.8%
Inter-assay (Run-to-Run)
n = 8; CV = 7.8%
Spiking Recovery
87,00%
Dilutation Linearity
91,00%
Crossreactivity
bovine Non-detectable
cat Yes (recommended dilution 1:4)
dog Non-detectable
goat Non-detectable
hamster Non-detectable
horse Non-detectable
mouse Non-detectable
pig Non-detectable
rabbit Non-detectable
rat Non-detectable
sheep Non-detectable
chicken Not tested
human Yes
monkey Yes (recommended dilution 1:4)
– Bekpinar S, Gurdol F, Unlucerci Y, Develi S, Yilmaz A. Serum levels of arginase I are associated with left ventricular function after myocardial infarction. Clin Biochem. 2011 Sep;44 (13):1090-3
– Dimitriades V, Rodriguez PC, Zabaleta J, Ochoa AC. Arginase I levels are decreased in the plasma of pediatric patients with atopic dermatitis. Ann Allergy Asthma Immunol. 2014 Sep;113 (3):271-5
– El-Hady SB, Farahat MH, Atfy M, Elhady MA. Nitric oxide metabolites and arginase I levels in beta-thalassemic patients: an Egyptian study. Ann Hematol. 2012 Aug;91 (8):1193-200
– Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother. 2011 Oct;60 (10):1419-30
– Giallongo C, Parrinello N, Tibullo D, La Cava P, Romano A, Chiarenza A, Barbagallo I, Palumbo GA, Stagno F, Vigneri P, Di Raimondo F. Myeloid derived suppressor cells (MDSCs) are increased and exert immunosuppressive activity together with polymorphonuclear leukocytes (PMNs) in chronic myeloid leukemia patients. PLoS One. 2014;9 (7):e101848
– Grasemann H, Schwiertz R, Grasemann C, Vester U, Racke K, Ratjen F. Decreased systemic bioavailability of L-arginine in patients with cystic fibrosis. Respir Res . Jun 9;7:87 (2006)
– Larkin SK, Morris CR, Styles LA, Kuypers FA. Elevated Plasma Arginase Levels in Hemoglobinopathies. Blood . 106 (11) (2005)
– Morris CR, Suh JH, Hagar W, Larkin S, Bland DA, Steinberg MH, Vichinsky EP, Shigenaga M, Ames B, Kuypers FA, Klings ES. Erythrocyte glutamine depletion, altered redox environment, and pulmonary hypertension in sickle cell disease. Blood. 2008 Jan 1;111 (1):402-10
– Nzoumbou-Boko R, Dethoua M, Gabriel F, Buguet A, Cespuglio R, Courtois P, Daulouede S, Bouteille B, Ngampo S, Mpandzou G, Semballa S, Vincendeau P. Serum arginase, a biomarker of treatment efficacy in human African trypanosomiasis. J Clin Microbiol. 2013 Jul;51 (7):2379-81
– Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R, Ochoa AC. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009 Feb 15;69 (4):1553-60
– Rotondo R, Barisione G, Mastracci L, Grossi F, Orengo AM, Costa R, Truini M, Fabbi M, Ferrini S, Barbieri O. IL-8 induces exocytosis of arginase 1 by neutrophil polymorphonuclears in nonsmall cell lung cancer. Int J Cancer. 2009 Aug 15;125 (4):887-93
– Rotondo R, Bertolotto M, Barisione G, Astigiano S, Mandruzzato S, Ottonello L, Dallegri F, Bronte V, Ferrini S, Barbieri O. Exocytosis of azurophil and arginase 1-containing granules by activated polymorphonuclear neutrophils is required to inhibit T lymphocyte proliferation. J Leukoc Biol. 2011 May;89 (5):721-7
– Scrimini S, Pons J, Agusti A, Soriano JB, Cosio BG, Torrecilla JA, Nunez B, Cordova R, Iglesias A, Jahn A, Crespi C, Sauleda J. Differential effects of smoking and COPD upon circulating myeloid derived suppressor cells. Respir Med. 2013 Dec;107 (12):1895-903
– Silva MA, Mirza DF, Buckels JA, Bramhall SR, Mayer D, Wigmore SJ, Murphy N, Richards DA. Arginine and urea metabolism in the liver graft: A study using microdialysis in human orthotopic liver transplantation. Transplantation . Nov 27;82(10):1304-11 (2006)
– Stejskal D, Vavrouskova J, Mares J, Urbanek K. Applications of new laboratory marker assays in neurological diagnoses – a pilot study. Biomed Pap Med Fac Univ Palack. 2005 Dec;149 (2):265-6
– Wu P, Wu D, Ni C, Ye J, Chen W, Hu G, Wang Z, Wang C, Zhang Z, Xia W, Chen Z, Wang K, Zhang T, Xu J, Han Y, Zhang T, Wu X, Wang J, Gong W, Zheng S, Qiu F, Yan J, Huang J. gammadeltaT17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity. 2014 May 15;40 (5):785-800
– Zahradka P, Wright B, Weighell W, Blewett H, Baldwin A, O K, Guzman RP, Taylor CG. Daily non-soy legume consumption reverses vascular impairment due to peripheral artery disease. Atherosclerosis. 2013 Oct;230 (2):310-4

