Apolipoprotein M Human ELISA
Human Apolipoprotein M (ApoM, protein G3a, NG20-like protein NG20), is a protein that in humans is encoded by the APOM gene. Two different isoforms have been found for this gene. ApoM is a secreted 25kDa member of the lipocalin protein family consisting of 188 amino acids. Human APOM shares sequence identity with pig (91%) and mouse (80%) APOM. The apolipoproteins are a structurally-unrelated group of proteins that all have some role in the transport of lipids in blood. Apolipoproteins plus phospholipids, cholesterol and triglycerides, form spherical particles with a lipid/hydrophobic center and a (apolipo)protein coat. The apolipoprotein coat promotes aqueous solubility and serves as a ligand for lipoprotein receptors. HDL may contain apoliporoteins A,C, D,E, J, L and M, while LDL contains apolipoproteins B and E.
Areas of investigation: Lipoprotein metabolism, Obesity, Atherosclerosis, Diabetes mellitus, Immune Response, Infection and Inflammation.
Type
Sandwich ELISA, Biotin-labelled antibody
Applications
Serum, Plasma (EDTA, citrate, heparin)
Sample Requirements
20 µl/well
Storage/Expiration
Store the complete kit at 2–8°C. Under these conditions, the kit is stable until the expiration date (see label on the box).
Calibration Curve
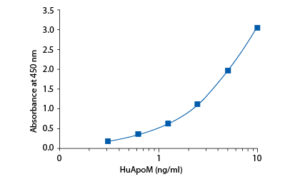 Calibration Range
Calibration Range
0.313–10 ng/ml
Limit of Detection
0.067 ng/ml
Intra-assay (Within-Run)
n = 8; CV = 5.1%
Inter-assay (Run-to-Run)
n = 5; CV = 5.7%
Spiking Recovery
107,60%
Dilutation Linearity
92,40%
– Engin-Ustun Y, Caglayan EK, Gocmen AY, Polat MF. Postmenopausal Osteoporosis Is Associated with Serum Chemerin and Irisin but Not with Apolipoprotein M Levels. J Menopausal Med. 2016 Aug;22 (2):76-9

