Albumin Rat ELISA
Albumin is mostly a simple hydrophilic protein present in cells and body fluids. Albumin is synthesized in the liver, and serum albumin (69 kDa, pI 4.9) occupies 56-60% of total serum proteins. Because of its large population, albumin is very important in maintaining plasma osmotic pressure. Albumin can bind hydrophobic physiological substances e.g. fatty acids, bilirubin, and thyroxine and contributes the transfer of these substances. Concentration of albumin in serum is lowered in liver cirrhosis, malnutrition, and pyrexial diseases due to decreased biosynthesis or increased consumption of albumin in, and also by secretion into urine in renal damage. In normal human subjects, excretion of albumin into urine is very little, about 30mg/day, but increased in glomerulonephritis, nephritic syndrome, and diabetic nephropathy. Urinary albumin sometimes increases in pyrexia, hypertension, congestive heart failure (CHF), and urinary tract infection (UTI). Even in healthy human, a transient upraise of urinary albumin is observed after hard exercise, muscle work, bathing in high temperature water, mental excitement, stress, intake of much protein, and before menstruation. These are called physiological or functional or sports proteinuria (albuminuria). Orthostatic proteinuria (albuminuria) is observed in teenagers. Rare hereditary analbuminemia in human is really albumin deficiency. A model analbuminemia rat has been established by Dr. Sumi Nagase which has been derived from Sprague-Dawley strain, and is called Nagase analbuminemia rat (NAR). Routine measurement of serum albumin is conveniently made with Shibayagi’s TIA assay kits for automatic analyzers. Shibayagi’s Albumin ELISA Kits can measure urinary albumin with high sensitivity, and are also applied to in vitro albumin biosynthesis system, checking of contamination with albumin in biological active substance preparations obtained by culture system, and liver transplantation experiments using NAR.
1. INTENDED USE
Rat Albumin ELISA Kit is a sandwich ELISA system for quantitative measurement of rat albumin. This is intended for research use only.
2. STORAGE AND EXPIRATION
When the complete kit is stored at 2-8°C, the kit is stable until the expiration date shown on the label on the box. Opened reagents should be used as soon as possible to avoid loss in optimal assay performance caused by storage environment.
3. ASSAY PRINCIPLE
In Shibayagi’s Rat Albumin ELISA Kit, standards or diluted samples are incubated in monoclonal anti-albumin antibody-coated wells to capture albumin. After 1 hour incubation and washing, HRP (horse radish peroxidase)-conjugated anti-albumin antibody is added, and incubated for 1 hour. After washing, HRP-complex remaining in wells is reacted with a chromogenic substrate (TMB) for 20 minutes, and reaction is stopped by addition of acidic solution, and absorbance of yellow product is measured spectrophotometrically at 450 nm. The absorbance is nearly proportional to albumin concentration. The standard curve is prepared by plotting absorbance against standard albumin concentrations. Albumin concentrations in unknown samples are determined using this standard curve.
4. ASSAY PROCEDURE
Remove the cover sheet of the antibody-coated plate after bringing up to room temperature.
- Wash the anti-albumin-coated plate (A) by filling the wells with washing buffer and discard 3 times(*②), then strike the plate upside-down onto folded several sheets of paper towel to remove residual buffer in the wells.
- Pipette 50 µl of buffer to each well and shake the plate gently on a plate shaker(*③).
- Pipette 5 µl of standard solution to the wells designated for standards, and 5 µl of properly diluted samples to the designated sample wells.
- Shake the plate gently on a plate shaker(*③).
- Stick a plate seal (*④) on the plate and incubate for 1 hour at 20-25oC.
- Discard the reaction mixture and rinse wells as step (1).
- Pipette 50 µl of HRP-conjugated anti-albumin antibody to all wells, and shake as step (4).
- Stick a plate seal (*④) on the plate and incubate the plate for 1 hour at 2025oC.
- Discard the reaction mixture and rinse wells as step (1).
- Pipette 50 µl of Chromogenic substrate reagent to wells, and shake as step (4).
- Stick a plate seal (*④) on the plate and incubate the plate for 20 minutes at 20-25oC.
- Add 50 µl of the reaction stopper to all wells to stop the coloration.
- Measure the absorbance of each well at 450 nm (reference wavelength, 620*nm) using a plate reader within 30 minutes.
5. CALCULATIONS
- Prepare a standard curve by plotting standard concentration on X-log and absorbance on Y-axis.
- Using the standard curve, read the albumin concentration of a sample at its absorbance*, and multiply the assay value by dilution factor if the sample has been diluted. Though the assay range is wide enough, in case the absorbance of some samples is higher than that of the highest standard, please repeat the assay after proper dilution of samples with the buffer solution.
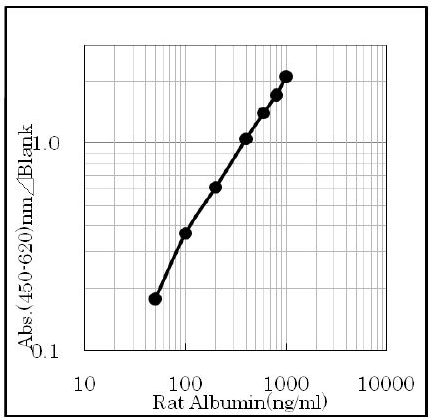
Assay range
The assay range of the kit is 50 ~ 1000 ng/ml.
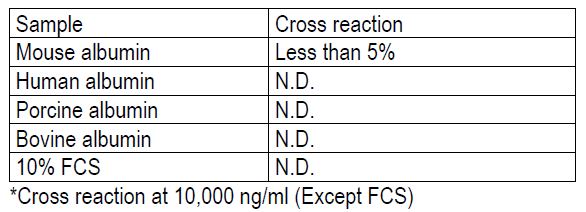
Specificity
The antibodies used in this kit are specific to albumin.
Precision of assay
Within assay variation (3 samples, 5 replicates assay), Mean CV is less than 5%
Reproducibility
Between assay variation (3 samples, 3 days, 3 replicates assay), Mean CV is less than 5%
Recovery test
Standard albumin was added in 3 concentrations to serum sample and assayed.
The recoveries were 93.7 ~98.9%
Dilution test
Serum sample was serially diluted by 3 steps.
The dilution curves showed linearity with R2 = 0.9951 and 0.9984.
– Emoto K, Tateno C, Hino H, Amano H, Imaoka Y, Asahina K, Asahara T, Yoshizato K. Efficient in vivo xenogeneic retroviral vector-mediated gene transduction into human hepatocytes. Hum Gene Ther. 2005 Oct;16 (10):1168-74
– Han KH, Kang YS, Han SY, Jee YH, Lee MH, Han JY, Kim HK, Kim YS, Cha DR. Spironolactone ameliorates renal injury and connective tissue growth factor expression in type II diabetic rats. Kidney Int. 2006 Jul;70 (1):111-20
– Han SY, Kim CH, Kim HS, Jee YH, Song HK, Lee MH, Han KH, Kim HK, Kang YS, Han JY, Kim YS, Cha DR. Spironolactone prevents diabetic nephropathy through an anti-inflammatory mechanism in type 2 diabetic rats. J Am Soc Nephrol. 2006 May;17 (5):1362-72
– Han SY, So GA, Jee YH, Han KH, Kang YS, Kim HK, Kang SW, Han DS, Han JY, Cha DR. Effect of retinoic acid in experimental diabetic nephropathy. Immunol Cell Biol. 2004 Dec;82 (6):568-76
– Hayashi K, Aoki T, Jin Z, Wang H, Nishino N, Kusano T, Yasuda D, Koizumi T, Enami Y, Odaira M, Yamada K, Mitamura K, Niiya T, Murai N, Kato H, Shimizu Y, Kusano M. Hepatocyte transplantation from steatotic liver in a rat model. J Surg Res. 2007 Sep;142 (1):104-12
– Ikegami-Kawai M, Okuda R, Nemoto T, Inada N, Takahashi T. Enhanced activity of serum and urinary hyaluronidases in streptozotocin-induced diabetic Wistar and GK rats. Glycobiology. 2004 Jan;14 (1):65-72
– Matsumoto N, Hiruma H, Nagaoka S, Fujiyama K, Kaneko A, Kawakami H. Cell processing on polyimide surface patterned by rubbing. Polymers for Advanced Technolo. 2008;19 (8):002-1008
– Odaira M, Aoki T, Miyamoto Y, Yasuhara R, Jin Z, Yu J, Nishino N, Yamada K, Kusano T, Hayashi K, Yasuda D, Koizumi T, Mitamura K, Enami Y, Niiya T, Murai N, Kato H, Shimizu Y, Kamijyo R, Kusano M. Cold preservation of the liver with oxygenation by a two-layer method. J Surg Res. 2009 Apr;152 (2):209-17
– Ohtake K, Ishiyama Y, Uchida H, Muraki E, Kobayashi J. Dietary nitrite inhibits early glomerular injury in streptozotocin-induced diabetic nephropathy in rats. Nitric Oxide. 2007 Sep;17 (2):75-81
– Suzuki YA, Tomoda M, Murata Y, Inui H, Sugiura M, Nakano Y. Antidiabetic effect of long-term supplementation with Siraitia grosvenori on the spontaneously diabetic Goto-Kakizaki rat. Br J Nutr. 2007 Apr;97 (4):770-5
– Ye SH, Watanabe J, Takai M, Iwasaki Y, Ishihara K. Design of functional hollow fiber membranes modified with phospholipid polymers for application in total hemopurification system. Biomaterials. 2005 Aug;26 (24):5032-41
– Yokoyama T, Ohashi K, Kuge H, Kanehiro H, Iwata H, Yamato M, Nakajima Y. In vivo engineering of metabolically active hepatic tissues in a neovascularized subcutaneous cavity. Am J Transplant. 2006 Jan;6 (1):50-9

