Adiponectin Mouse HEK293
Adiponectin, also referred to as Acrp30, AdipoQ and GBP-28, is a recently discovered 244 aminoacid protein, the product of the apM1 gene, which is physiologically active and specifically and highly expressed in adipose cells. The protein belongs to the soluble defence collagen superfamily; it has a collagen-like domain structurally homologous with collagen VIII and X and complement factor C1q-like globular domain. Adiponectin forms homotrimers, which are the building blocks for higher order complexes found circulating in serum. Together, these complexes make up approximately 0.01% of total serum protein. Adiponectin receptors AdipoR1 and AdipoR2 have been recently cloned; AdipoR1 is abundantly expressed in skeletal muscle, whereas AdipoR2 is predominantly expressed in the liver. Paradoxically, adipose tissue-expressed adiponectin levels are inversely related to the degree of adiposity. Adiponectin concentrations correlate negatively with glucose, insulin, triglyceride concentrations, liver fat content and body mass index and positively with high-density lipoprotein-cholesterol levels, hepatic insulin sensitivity and insulin-stimulated glucose disposal. Adiponectin has been shown to increase insulin sensitivity and decrease plasma glucose by increasing tissue fat oxidation. Of particular interest is that low adiponectin serum levels predict type 2 diabetes independent of other risk factors. Adiponectin also inhibits the inflammatory processes of atherosclerosis suppressing the expression of adhesion and cytokine molecules in vascular endothelial cells and macrophages, respectively. This adipokine plays a role as a scaffold of newly formed collagen in myocardial remodelling after ischaemic injury and also stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signalling in endothelial cells. Low serum adiponectin levels are found in patients with coronary artery disease. Moreover, high circulating levels of adiponectin are associated with decreased risk of myocardial infarction, independent of other factors. Altogether, adiponectin has the potential to become a clinically relevant parameter to be measured routinely in subjects at risk for type 2 diabetes, atherosclerosis and the metabolic syndrome.
Type
Recombinant
Source
HEK293
Purity
Purity as determined by densitometric image analysis: >98%
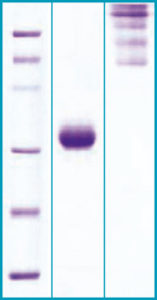
SDS-PAGE Gel
12% SDS-PAGE separation of Mouse A diponectin
1. M.W. marker – 14, 21, 31, 45, 66, 97 kDa
2. reduced and boiled sample, 5μg/lane
3. non-reduced and non-boiled sample, 5μg/lane
Biological Activity
Full-length adiponectin has been shown to activate AMP-activated protein kinase in hepatocyte. It can also activate AMPK in HepG2 human hepatocytes at the concentration of as low as 1.0 µg/ml. In vitro gluconeogenesis assay in primary rat hepatocytes was performed, showing the murine adiponectin derived from mammalian cells can inhibit glucose production.
Endotoxin
< 0.1 EU/ug
Formulation
Filtered (0,4 μm) and lyophilized in 0.5 mg/mL in 0.05 M phosphate buffer, 0.075 M NaCl, pH 7.4
Reconstitution
Add deionized water to prepare a working stock solution of approximately 0.5 mg/mL and let the lyophilized pellet dissolve completely. Product is not sterile! Filter your culture media/working solutions containing this product before using in cell culture.
Applications
Western blotting, ELISA, Cell culture and/or animal studies
Shipping
At ambient temperature. Upon receipt, store the product at the temperature recommended below.
Storage/Expiration
Store the lyophilized protein at –80 °C. Lyophilized protein remains stable until the expiry date when stored at –80 °C. Aliquot reconstituted protein to avoid repeated freezing/thawing cycles and store at –80 °C for long term storage. Reconstituted protein can be stored at 4 °C for a week.
Quality Control Test
BCA to determine quantity of the protein.
SDS PAGE to determine purity of the protein.
GFC to determine purity of the protein.
LAL to determine quantity of endotoxin.
– Berner HS, Lyngstadaas SP, Spahr A, Monjo M, Thommesen L, Drevon CA, Syversen U, Reseland JE. Adiponectin and its receptors are expressed in bone-forming cells. Bone . Oct;35(4):842-9 (2004)
– Conde J, Gomez R, Bianco G, Scotece M, Lear P, Dieguez C, Gomez-Reino J, Lago F, Gualillo O. Expanding the adipokine network in cartilage: identification and regulation of novel factors in human and murine chondrocytes. Ann Rheum Dis. 2011 Mar;70 (3):551-9
– Elfeky M, Kaede R, Okamatsu–Ogura Y, Kimura K. Adiponectin inhibits LPS-induced HMGB1 release through an AMP kinase- and heme oxygenase-1-dependent pathway in RAW 264 macrophage cells. Mediators of Inflammation. May 2016;
– Medoff BD, Okamoto Y, Leyton P, Weng M, Sandall BP, Raher MJ, Kihara S, Bloch KD, Libby P, Luster AD. Adiponectin deficiency increases allergic airway inflammation and pulmonary vascular remodeling. Am J Respir Cell Mol Biol. 2009 Oct;41 (4):397-406
– Nakanishi K, Takeda Y, Tetsumoto S, Iwasaki T, Tsujino K, Kuhara H, Jin Y, Nagatomo I, Kida H, Goya S, Kijima T, Maeda N, Funahashi T, Shimomura I, Tachibana I, Kawase I. Involvement of endothelial apoptosis underlying chronic obstructive pulmonary disease-like phenotype in adiponectin-null mice: implications for therapy. Am J Respir Crit Care Med. 2011 May 1;183 (9):1164-75
– Parker-Duffen JL, Nakamura K, Silver M, Kikuchi R, Tigges U, Yoshida S, Denzel MS, Ranscht B, Walsh K. T-cadherin is essential for adiponectin-mediated revascularization. J Biol Chem. 2013 Aug 23;288 (34):24886-97
– Shore SA, Terry RD, Flynt L, Xu A, Hug C. Adiponectin attenuates allergen-induced airway inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol. 2006 Aug;118 (2):389-95
– Teoh H, Quan A, Bang KW, Wang G, Lovren F, Vu V, Haitsma JJ, Szmitko PE, Al-Omran M, Wang CH, Gupta M, Peterson MD, Zhang H, Chan L, Freedman J, Sweeney G, Verma S. Adiponectin deficiency promotes endothelial activation and profoundly exacerbates sepsis-related mortality. Am J Physiol Endocrinol Metab. 2008 Sep;295 (3):E658-64
– Wang Y, Lam JB, Lam KS, Liu J, Lam MC, Hoo RL, Wu D, Cooper GJ, Xu A. Adiponectin modulates the glycogen synthase kinase-3beta/beta-catenin signaling pathway and attenuates mammary tumorigenesis of MDA-MB-231 cells in nude mice. Cancer Res. 2006 Dec 1;66 (23):11462-70
– Wang Y, Lam KS, Xu JY, Lu G, Xu LY, Cooper GJ, Xu A. Adiponectin inhibits cell proliferation by interacting with several growth factors in an oligomerization-dependent manner. J Biol Chem . May 6;280(18):18341-7 (2005)
– Xu A, Yin S, Wong L, Chan KW, Lam KS. Adiponectin ameliorates dyslipidemia induced by the human immunodeficiency virus protease inhibitor ritonavir in mice. Endocrinology. 2004 Feb;145 (2):487-94

