hs-Troponin I Assay
Diazyme's hs-Troponin I (hs-cTnI) Assay is for the quantitative determination of cardiac troponin I in serum samples. The analyte should be measured according to specific application parameters for specific chemistry analyzers. Diazyme's hs-Troponin I assay kit consists of two liquid stable reagents and the calibrator and control are packaged separately.
Diazyme’s hs-Troponin I (hs-cTnI) Assay is for the quantitative determination of cardiac troponin I in serum samples. The analyte should be measured according to specific application parameters for specific chemistry analyzers. Diazyme’s hs-Troponin I assay kit consists of two liquid stable reagents and the calibrator and control are packaged separately.
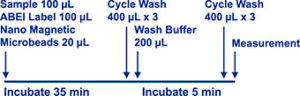
The hs-cTnI Assay is a sandwich immunoluminescent assay that uses an anti-Troponin I monoclonal antibody labeled with ABEI, magnetic microbeads coated with an anti-Troponin I monoclonal antibody. Sample, calibrator or control, along with ABEI Label and magnetic microbeads are mixed thoroughly and incubated at 37° to form a sandwich complex. After sedimentation in a magnetic field, the sandwich is then washed to remove free ABEI labeled antibody and other unbound substances. Subsequently, the starter reagents are added and a flash chemiluminescent reaction is initiated. The light signal is measured by a photomultiplier as RLU within 3 seconds and the signal is proportional to the concentration of Troponin I present in the samples.