Potassium Assay
For in vitro quantitative determination of potassium in human serum.
Potassium Assay has an outstanding linearity range from 2.0 mmol/L – 8.0 mmol/L. Potassium Assay offers excellent precision CV% of <2%. A comparison study showed that the assay has good correlation with existing ISE method’s with a correlation coefficient value of 0.98, slope of 1.07 and y intercept of -0.30. The assay shows virtually no affect by the following interfering substances at indicated concentrations: Na+ 150mM, NH4+ 0.5 mM, Ca2+ 7.5 mM, Pi 2.0 mM, ascorbic acid 10.0 mM, Zn2+ 0.5 mM, Fe3+ 0.5 mM, Cu2+ 0.5 mM, triglycerides 1000 mg/dL, hemoglobin 500 mg/dL, conjugated bilirubin 20 mg/ dL and unconjugated bilirubin 15 mg/dL.
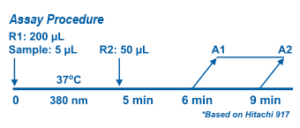
Potassium is determined spectrophotometrically through a kinetic coupling assay system using potassium dependent pyruvate kinase. Pyruvate generated is converted to lactate accompanying conversion of NADH analog to NAD analog. The corresponding decrease of optical density at 380 nm is proportional to the potassium concentration in the serum.