Direct Enzymatic HbA1c
Direct Enzymatic Hemoglobin A1c reagents are intended for use in the quantitative determination of stable HbA1c in human whole blood samples.
IFCC certified Direct Enzymatic HbA1c Assay has no interference from hemoglobin variants including HbC, HbS, HbE, and caramylated acetylated Hb or labile HbA1c. This unique freedom from interference combined with unparalleled precision (intra and inter % CV values less than 2.6%) means that Diazyme’s Direct Enzymatic HbA1c Assay provides unique performance advantages over conventional immunoassay and chromatography methods. Patented enzymatic method measures HbA1c directly using only a single instrument channel and completely eliminates the latex particles cuvette contamination often seen in HbA1c immunoassay methods. This reduces instrument maintenance requirements. Diazyme’s Direct Enzymatic HbA1c can be used on most automated chemistry analyzers and reagent transfer can be eliminated for most chemistry systems with instrument specific packaging options including RocheTM Hitachi 917 series, Beckman AU (400/600/640/680), Beckman Synchron CX, LX and DXC analyzers. The assay correlates well with conventional methods such as the Tosoh™ HPLC and Roche™ Tina-Quant methods.
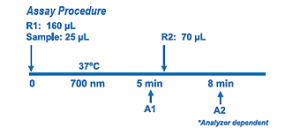
Enzymatic HbA1c Assay is an enzymatic assay in which lysed whole blood samples are subjected to extensive protease digestion with Bacillus sp protease. This process releases amino acids including glycated valines from the hemoglobin beta chains. Glycated valines then serve as substrates for specific recombinant fructosyl valine oxidase (FVO) enzyme, produced in E. coli. The recombinant FVO specifically cleaves N-terminal valines and produces hydrogen peroxide. This, in turn, is measured using a horseradish peroxidase (POD) catalyzed reaction and a suitable chromogen. No separate measurement for total hemoglobin (Hb) is needed in this Direct Enzymatic HbA1c Assay. The HbA1c concentration is expressed directly as %HbA1c by use of a suitable calibration curve in which the calibrators have values for each level in %HbA1c.