Myoglobin
Myoglobin Assay is for the quantitative determination of myoglobin in human serum and plasma.
Myoglobin Assay is based on latex enhanced immunoturbidimetric method. The assay is formulated in a convenient dual liquid stable format, and it is highly cost effective. Myoglobin is a cardiac biomarker that helps diagnose or rule out heart attacks. An increase in myoglobin is detectable sooner than troponin I, but is not as specific for heart damage and it will not stay elevated as long as troponin I. Although a negative myoglobin result effectively rules out a heart attack, a positive result must be confirmed by additional testing. Myoglobin Assay is linear from 13.2 – 615.9 ng/ml with a correlation coefficient value of 0.9972, slope of 0.959 and y intercept of -5.141. The common substances of triglyceride, ascorbic acid, bilirubin, conjugated bilirubin, hemoglobin, rheumatoid factor, heparin and additional substances of drug interference showed no significant interference.
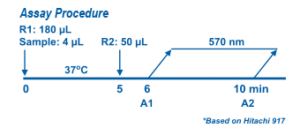
Myoglobin Assay is based on a latex enhanced immunoturbidimetric assay. When an antigen-antibody reaction occurs between myoglobin in a sample and anti-myoglobin antibodies which have been sensitized to latex particles, agglutination occurs. This agglutination is detected as an absorbance change (570 nm), with the magnitude of the change being proportional to the quantity of myoglobin in the sample. The actual concentration is then determined from a calibration curve prepared from calibrators of known concentrations.