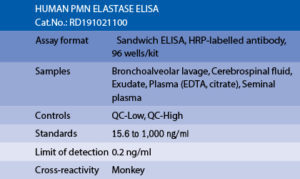
PMN Elastase Human ELISA
The human organism reacts with an inflammatory response to attacks of invading pathogens (micro-organisms and viruses) or damaged tissue (after accidents or surgery). Polymorphonuclear (PMN) granulocytes play an important role as primary defence cells in this inflammatory reaction. Different bloodstream mediators (cytokines, leukotrienes, complement factors, bacterial endotoxins, clotting and fibrinolysis factors) attract and stimulate these cells to phagocytize and destroy not naturally occurring agents. PMN granulocytes use proteinases to digest these agents and tissue debris. One of these proteinases is PMN elastase which is localised in the azurophilic granules of the polymorphonuclear granulocytes. During phagocytosis of foreign substances these enzymes are also partially excreted into the extracellular surrounding, where the aktivity of PMN elastase is regulated by inhibitors (esp. the α1-proteinase inhibitor, α1-PI). An overwhelming release of PMN elastase, however, can exceed the inhibitory potential of the α1-proteinase inhibitor. Thus, enzymatically active PMN elastase, together with simultaneously produced oxidants (O2-radicals, H2O2, OH-radicals), can cause local tissue injury. Due to the bloodstream and lymphatic system, however, α1-PI is delivered subsequently and eventually able to form a complex with all excreted elastase. Therefore, the concentration of the PMN elastase/ α1-PI complex correlates with the released PMN elastase and can be used as a measure for the activity of granulocytes during an inflammatory response. Primarily, determinations of PMN elastase find its application in observation of the course of trauma, shock and sepsis. Further indications are the areas of hemodialysis, infections by obstetrics, joint diseases, effusions of sport injuries, intestinal affection, pancreatitis, cystic fibrosis and male adnex affections.
The RD191021100 Human PMN Elastase ELISA is a sandwich enzyme immunoassay for the quantitative measurement of the complex of human PMN elastase and α1-proteinase inhibitor (α1-PI) in plasma.
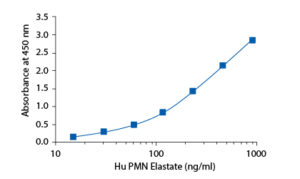
In the BioVendor Human PMN Elastase ELISA, Standards, Quality Controls and samples are incubated in microplate wells pre-coated with polyclonal antibody. After 60 minutes incubation and washing, polyclonal antibody, conjugated with horseradish peroxidase (HRP) is added to the wells and incubated for 60 minutes with captured PMN elastase/α1-PI complex. Following another washing step, the remaining HRP conjugate is allowed to react with the substrate solution (TMB). The reaction is stopped by addition of acidic solution and absorbance of the resulting yellow product is measured. The absorbance is proportional to the concentration of PMN elastase. A standard curve is constructed by plotting absorbance values against concentrations of Standards, and concentrations of unknown samples are determined using this standard curve.
Intended use
Clinical Application
Test principle
Summary of protocol
– Allan EK, Holyoake TL, Jorgensen HG. . – Chimenti L, Morici G, Paterno A, Bonanno A, Vultaggio M, Bellia V, Bonsignore MR. Environmental conditions, air pollutants, and airway cells in runners: a longitudinal field study. J Sports Sci. 2009 Jul;27 (9):925-35
– Laing SJ, Jackson AR, Walters R, Lloyd-Jones E, Whitham M, Maassen N, Walsh NP. Human blood neutrophil responses to prolonged exercise with and without a thermal clamp. J Appl Physiol. 2008 Jan;104 (1):20-6
– Lisowska-Myjak B, Zytynska-Daniluk J, Skarzynska E. Concentrations of neutrophil-derived proteins in meconium and their correlations. Biomark Med. 2016 Aug;10 (8):819-29
– Naskalski JW, Kapusta M, Fedak D, Dumnicka P, Kusnierz-Cabala B, Kuzniewski M, Sulowicz W. Effect of Hemodialysis on Acid Leukocyte-Type Ribonuclease, Alkaline Ribonuclease and Polymorphonuclear Elastase Serum Levels in Patients with End-Stage Renal Disease. Nephron Clin Pract. 2009 Jun 16;112 (4):c248-c254
– Park CJ, Clark SG, Lichtensteiger CA, Jamison RD, Johnson AJ. Accelerated wound closure of pressure ulcers in aged mice by chitosan scaffolds with and without bFGF. Acta Biomater. 2009 Jul;5 (6):1926-36
– Pawlica-Gosiewska D, Solnica B, Gawlik K, Cibor D, Mach T, Fedak D, Owczarek D. The use of selected neutrophil protein plasma concentrations in the diagnosis of Crohn’s disease and ulcerative colitis – a preliminary report. Postepy Hig Med Dosw (Online). 2017 Apr 6;71(0):243-253

