Clusterin Human HEK293
Clusterin is a 75–80 kD disulfide-linked heterodimeric protein containing about 30% of N-linked carbohydrate rich in sialic acid, but truncated forms targeted to the nucleus have also been identified.
The precursor polypeptide chain is cleaved proteolytically to remove the 22-mer secretory signal peptide and subsequently between residues 227/228 to generate the alpha and beta chains. These are assembled anti-parallel to give a heterodimeric molecule in which the cysteine-rich centers are linked by five disulfide bridges and are flanked by two predicted coiled-coil alpha-helices and three predicted amphipathic alpha-helices. Clusterin is a heavily N-glycosylated protein.
Across a broad range of species clusterin shows 70% to 80% of sequence homology. It is ubiquitously expressed in most mammalian tissues and can be found in plasma, milk, urine, cerebrospinal fluid and semen.
It is able to bind and form complexes with numerous partners such as immunoglobulins, lipids, heparin, bacteria, complement components, paraoxonase, beta amyloid, leptin and others. Clusterin has been ascribed a plethora of functions such as phagocyte recruitment, aggregation induction, complement attack prevention, apoptosis inhibition, membrane remodelling, lipid transport, hormone transport and/or scavenging, matrix metalloproteinase inhibition.
A detailed mechanism of clusterin has not been defined. One tempting hypothesis says that clusterin is an extracellular chaperone protecting cells from stress induced by degraded and misfolded protein precipitates.Clusterin is up- or downregulated on the mRNA or protein level in many pathological and clinically relevant situations including cancer, organ regeneration, infection, Alzheimer disease, retinitis pigmentosa, myocardial infarction, renal tubular damage, autoimmunity and others.
Type
Recombinant
Description
Total 438 AA, M.w. 51.27kDa (calculated). The AA sequence (AA 1–427) is identical to Swiss-Prot-P10909 (AA 23–449, secreted Human Clusterin). C-terminal Flag-tag, 11 extra AA (highlighted).
Source
HEK293
Purity
Purity as determined by densitometric image analysis: >90%
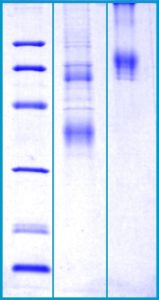
SDS-PAGE Gel
12% SDS-PAGE separation of Human Clusterin
1. M.W. marker – 14, 21, 31, 45, 66, 97 kDa
2. reduced and heated sample, 5μg/lane
3. non-reduced and non-heated sample, 5μg/lane
Endotoxin
< 1.0 EU/ug
Formulation
Filtered (0.4 μm) and lyophilized from 0.5 mg/ml in 20mM Tris buffer, 50mM NaCl, pH 7.5
Reconstitution
Add deionized water to prepare a working stock solution of approximately 0.5 mg/mL and let the lyophilized pellet dissolve completely. Product is not sterile! Please filter the product by an appropriate sterile filter before using it in the cell culture.
Applications
Western blotting, ELISA, Cell culture and/or animal studies
Shipping
At ambient temperature. Upon receipt, store the product at the temperature recommended below.
Storage/Expiration
Store the lyophilized protein at –80 °C. Lyophilized protein remains stable until the expiry date when stored at –80 °C. Aliquot reconstituted protein to avoid repeated freezing/thawing cycles and store at –80 °C for long term storage. Reconstituted protein can be stored at 4 °C for a week.
Quality Control Test
BCA to determine quantity of the protein.
SDS PAGE to determine purity of the protein.
LAL to determine quantity of endotoxin.
– Cheung MC, Vaisar T, Han X, Heinecke JW, Albers JJ. Phospholipid transfer protein in human plasma associates with proteins linked to immunity and inflammation. Biochemistry. 2010 Aug 31;49 (34):7314-22
– Kliger Y, Levy O, Oren A, Ashkenazy H, Tiran Z, Novik A, Rosenberg A, Amir A, Wool A, Toporik A, Schreiber E, Eshel D, Levine Z, Cohen Y, Nold-Petry C, Dinarello CA, Borukhov I. Peptides modulating conformational changes in secreted chaperones: from in silico design to preclinical proof of concept. Proc Natl Acad Sci U S A. 2009 Aug 18;106 (33):13797-801
– Zhang F, Sha J, Wood TG, Galindo CL, Garner HR, Burkart MF, Suarez G, Sierra JC, Agar SL, Peterson JW, Chopra AK. Alteration in the activation state of new inflammation-associated targets by phospholipase A2-activating protein (PLAA). Cell Signal. 2008 May;20 (5):844-61
– Zhang F, Suarez G, Sha J, Sierra JC, Peterson JW, Chopra AK. Phospholipase A2-activating protein (PLAA) enhances cisplatin-induced apoptosis in HeLa cells. Cell Signal. 2009 Jul;21 (7):1085-99

