Adiponectin Human HEK293
Adiponectin, also referred to as Acrp30, AdipoQ and GBP-28, is a recently discovered 244 aminoacid protein, the product of the apM1 gene, which is physiologically active and specifically and highly expressed in adipose cells. The protein belongs to the soluble defence collagen superfamily; it has a collagen-like domain structurally homologous with collagen VIII and X and complement factor C1q-like globular domain. Adiponectin forms homotrimers, which are the building blocks for higher order complexes found circulating in serum. Together, these complexes make up approximately 0.01% of total serum protein. Adiponectin receptors AdipoR1 and AdipoR2 have been recently cloned; AdipoR1 is abundantly expressed in skeletal muscle, whereas AdipoR2 is predominantly expressed in the liver. Paradoxically, adipose tissue-expressed adiponectin levels are inversely related to the degree of adiposity. Adiponectin concentrations correlate negatively with glucose, insulin, triglyceride concentrations, liver fat content and body mass index and positively with high-density lipoprotein-cholesterol levels, hepatic insulin sensitivity and insulin-stimulated glucose disposal. Adiponectin has been shown to increase insulin sensitivity and decrease plasma glucose by increasing tissue fat oxidation. Of particular interest is that low adiponectin serum levels predict type 2 diabetes independent of other risk factors. Adiponectin also inhibits the inflammatory processes of atherosclerosis suppressing the expression of adhesion and cytokine molecules in vascular endothelial cells and macrophages, respectively. This adipokine plays a role as a scaffold of newly formed collagen in myocardial remodelling after ischaemic injury and also stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signalling in endothelial cells. Low serum adiponectin levels are found in patients with coronary artery disease. Moreover, high circulating levels of adiponectin are associated with decreased risk of myocardial infarction, independent of other factors. Altogether, adiponectin has the potential to become a clinically relevant parameter to be measured routinely in subjects at risk for type 2 diabetes, atherosclerosis and the metabolic syndrome.
Type
Recombinant
Source
HEK293
Purity
C-terminal flag tag
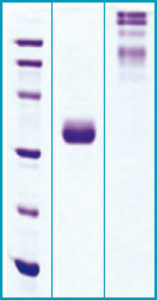
SDS-PAGE Gel
12% SDS-PAGE separation of Human A diponectin
- M.w. marker – 14, 21, 31, 45, 66, 97 kDa
- reduced and heated sample, 5μg/lane
- non-reduced and non-heated sample, 5μg/lane
Biological Activity
In vitro gluconeogenesis assay in primary hepatocytes was performed, showing the human adiponectin derived from mammalian cells can inhibit glucose production. The ED50 was ~6 µg/ml.
Endotoxin
< 0.1 EU/ug
Formulation
Filtered (0,4 μm) and lyophilized in 0.5 mg/mL in 0.05 M phosphate buffer, 0.075 M NaCl, pH 7.4
Reconstitution
Add deionized water to prepare a working stock solution of approximately 0.5 mg/mL and let the lyophilized pellet dissolve completely. Product is not sterile! Filter your culture media/working solutions containing this product before using in cell culture.
Applications
Western blotting, ELISA, Cell culture and/or animal studies
Shipping
At ambient temperature. Upon receipt, store the product at the temperature recommended below.
Storage/Expiration
Store the lyophilized protein at –80 °C. Lyophilized protein remains stable until the expiry date when stored at –80 °C. Aliquot reconstituted protein to avoid repeated freezing/thawing cycles and store at –80 °C for long term storage. Reconstituted protein can be stored at 4 °C for a week.
Quality Control Test
BCA to determine quantity of the protein.
SDS PAGE to determine purity of the protein.
GFC to determine purity of the protein.
LAL to determine quantity of endotoxin.
| – Berger E, Rome S, Vega N, Ciancia C, Vidal H. Transcriptome profiling in response to adiponectin in human cancer-derived cells. Physiol Genomics. 2010 Sep;42A (1):61-70
– Conde J, Gomez R, Bianco G, Scotece M, Lear P, Dieguez C, Gomez-Reino J, Lago F, Gualillo O. Expanding the adipokine network in cartilage: identification and regulation of novel factors in human and murine chondrocytes. Ann Rheum Dis. 2011 Mar;70 (3):551-9 – Folco EJ, Rocha VZ, Lopez-Ilasaca M, Libby P. Adiponectin inhibits pro-inflammatory signaling in human macrophages independent of interleukin-10. J Biol Chem. 2009 Sep 18;284 (38):25569-75 – Greenstein AS, Khavandi K, Withers SB, Sonoyama K, Clancy O, Jeziorska M, Laing I, Yates AP, Pemberton PW, Malik RA, Heagerty AM. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation. 2009 Mar 31;119 (12):1661-70 – Haugen F, Drevon CA. Activation of nuclear factor-kappaB by high molecular weight and globular adiponectin. Endocrinology. 2007 Nov;148 (11):5478-86 – Jalovaara K, Santaniemi M, Timonen M, Jokelainen J, Kesaniemi YA, Ukkola O, Keinanen-Kiukaanniemi S, Rajala U. Low serum adiponectin level as a predictor of impaired glucose regulation and type 2 diabetes mellitus in a middle-aged Finnish population. Metabolism. 2008 Aug;57 (8):1130-4 – Kamio N, Akifusa S, Yamaguchi N, Yamashita Y. Induction of granulocyte colony-stimulating factor by globular adiponectin via the MEK-ERK pathway. Mol Cell Endocrinol. 2008 Sep 24;292 (1-2):20-5 – Kitajima K, Miura S, Yamauchi T, Uehara Y, Kiya Y, Rye KA, Kadowaki T, Saku K. Possibility of increasing cholesterol efflux by adiponectin and its receptors through the ATP binding cassette transporter A1 in HEK293T cells. Biochem Biophys Res Commun. 2011 Jul 29;411 (2):305-11 – Lamers D, Famulla S, Wronkowitz N, Hartwig S, Lehr S, Ouwens DM, Eckardt K, Kaufman JM, Ryden M, Muller S, Hanisch FG, Ruige J, Arner P, Sell H, Eckel J. Dipeptidyl Peptidase 4 Is a Novel Adipokine Potentially Linking Obesity to the Metabolic Syndrome. Diabetes. 2011 May 20; – Mahadev K, Wu X, Donnelly S, Ouedraogo R, Eckhart AD, Goldstein BJ. Adiponectin inhibits vascular endothelial growth factor-induced migration of human coronary artery endothelial cells. Cardiovasc Res. 2008 May 1;78 (2):376-84 – Maillard V, Uzbekova S, Guignot F, Perreau C, Rame C, Coyral-Castel S, Dupont J. Effect of adiponectin on bovine granulosa cell steroidogenesis, oocyte maturation and embryo development. Reprod Biol Endocrinol. 2010;8:23 – Murdolo G, Hammarstedt A, Schmelz M, Jansson PA, Smith U. Acute hyperinsulinemia differentially regulates interstitial and circulating adiponectin oligomeric pattern in lean and insulin-resistant, obese individuals. J Clin Endocrinol Metab. 2009 Nov;94 (11):4508-16 – Nigro E, Scudiero O, Sarnataro D, Mazzarella G, Sofia M, Bianco A, Daniele A. Adiponectin affects lung epithelial A549 cell viability counteracting TNFalpha and IL-1ss toxicity through AdipoR1. Int J Biochem Cell Biol. 2013 Jun;45 (6):1145-53 – Pisto P, Ukkola O, Santaniemi M, Kesaniemi YA. Plasma adiponectin-an independent indicator of liver fat accumulation. Metabolism. 2011 May 10; – Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R, Ochoa AC. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009 Feb 15;69 (4):1553-60 – Sell H, Laurencikiene J, Taube A, Eckardt K, Cramer A, Horrighs A, Arner P, Eckel J. Chemerin is a novel adipocyte-derived factor inducing insulin resistance in primary human skeletal muscle cells. Diabetes. 2009 Dec;58 (12):2731-40 – Stejskal D, Proskova J, Solichova P. Adiponectin added into the plasma of healthy probands does not affect platelet aggregability. Biomed Pap Med Fac Univ Palack. 2006 Jul;150 (1):89-90 |

