Adiponectin (HEK) Human, Sheep Polyclonal Antibody
Adiponectin, also referred to as Acrp30, AdipoQ and GBP-28, is a recently discovered 244 aminoacid protein, the product of the apM1 gene, which is physiologically active and specifically and highly expressed in adipose cells. The protein belongs to the soluble defence collagen superfamily; it has a collagen-like domain structurally homologous with collagen VIII and X and complement factor C1q-like globular domain. Adiponectin forms homotrimers, which are the building blocks for higher order complexes found circulating in serum. Together, these complexes make up approximately 0.01% of total serum protein. Adiponectin receptors AdipoR1 and AdipoR2 have been recently cloned; AdipoR1 is abundantly expressed in skeletal muscle, whereas AdipoR2 is predominantly expressed in the liver. Paradoxically, adipose tissue-expressed adiponectin levels are inversely related to the degree of adiposity. Adiponectin concentrations correlate negatively with glucose, insulin, triglyceride concentrations, liver fat content and body mass index and positively with high-density lipoprotein-cholesterol levels, hepatic insulin sensitivity and insulin-stimulated glucose disposal. Adiponectin has been shown to increase insulin sensitivity and decrease plasma glucose by increasing tissue fat oxidation. Of particular interest is that low adiponectin serum levels predict type 2 diabetes independent of other risk factors. Adiponectin also inhibits the inflammatory processes of atherosclerosis suppressing the expression of adhesion and cytokine molecules in vascular endothelial cells and macrophages, respectively. This adipokine plays a role as a scaffold of newly formed collagen in myocardial remodelling after ischaemic injury and also stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signalling in endothelial cells. Low serum adiponectin levels are found in patients with coronary artery disease. Moreover, high circulating levels of adiponectin are associated with decreased risk of myocardial infarction, independent of other factors. Altogether, adiponectin has the potential to become a clinically relevant parameter to be measured routinely in subjects at risk for type 2 diabetes, atherosclerosis and the metabolic syndrome.
Type
Polyclonal Antibody
Applications
Western blotting, ELISA, Immunohistochemistry
Antibodies Applications
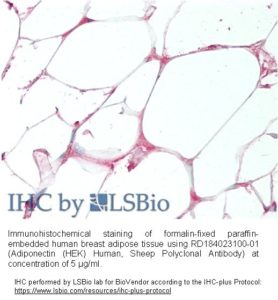
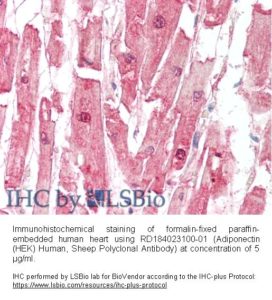
Source of Antigen
HEK293
Hosts
Sheep
Preparation
The antibody was raised in sheep by immunization with the recombinant Human Adiponectin.
Amino Acid Sequence
Glu 1 to Gln 5 were confirmed by N-terminal sequencing
Species Reactivity
Human. Not yet tested in other species.
Purification Method
C-terminal flag tag
Antibody Content
0.1 mg (determined by BCA method, BSA was used as a standard)
Formulation
The antibody is lyophilized in 0.05 M phosphate buffer, 0.1 M NaCl, pH 7.2. AZIDE FREE.
Reconstitution
Add 0.1 ml of deionized water and let the lyophilized pellet dissolve completely. Slight turbidity may occur after reconstitution, which does not affect activity of the antibody. In this case clarify the solution by centrifugation.
Shipping
At ambient temperature. Upon receipt, store the product at the temperature recommended below.
Storage/Expiration
The lyophilized antibody remains stable and fully active until the expiry date when stored at –20°C. Aliquot the product after reconstitution to avoid repeated freezing/thawing cycles and store frozen at –80°C. Reconstituted antibody can be stored at 4°C for a limited period of time; it does not show decline in activity after one week at 4°C.
Quality Control Test
Indirect ELISA – to determine titer of the antibody
SDS PAGE – to determine purity of the antibody
Note
This product is for research use only.
– Adachi M, Brenner DA. High molecular weight adiponectin inhibits proliferation of hepatic stellate cells via activation of adenosine monophosphate-activated protein kinase. Hepatology. 2008 Feb;47 (2):677-85
– Andersen KK, Frystyk J, Wolthers OD, Heuck C, Flyvbjerg A. Gender differences of oligomers and total adiponectin during puberty: a cross-sectional study of 859 Danish school children. J Clin Endocrinol Metab. 2007 May;92 (5):1857-62
– Araki S, Dobashi K, Kubo K, Asayama K, Shirahata A. High molecular weight, rather than total, adiponectin levels better reflect metabolic abnormalities associated with childhood obesity. J Clin Endocrinol Metab. 2006 Dec;91 (12):5113-6
– Asada K, Yoshiji H, Noguchi R, Ikenaka Y, Kitade M, Kaji K, Yoshii J, Yanase K, Namisaki T, Yamazaki M, Tsujimoto T, Akahane T, Uemura M, Fukui H. Crosstalk between high-molecular-weight adiponectin and T-cadherin during liver fibrosis development in rats. Int J Mol Med. 2007 Nov;20 (5):725-9
– Basu R, Pajvani UB, Rizza RA, Scherer PE. Selective downregulation of the high molecular weight form of adiponectin in hyperinsulinemia and in type 2 diabetes: differential regulation from nondiabetic subjects. Diabetes. 2007 Aug;56 (8):2174-7
– Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001; 7:947-953.
– Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab. 2002;13:84-89.
– Beulens JW, van Loon LJ, Kok FJ, Pelsers M, Bobbert T, Spranger J, Helander A, Hendriks HF. The effect of moderate alcohol consumption on adiponectin oligomers and muscle oxidative capacity: a human intervention study. Diabetologia. 2007 Jul;50 (7):1388-92
– Bluher M, Brennan AM, Kelesidis T, Kratzsch J, Fasshauer M, Kralisch S, Williams CJ, Mantzoros CS. Total and high-molecular weight adiponectin in relation to metabolic variables at baseline and in response to an exercise treatment program: comparative evaluation of three assays. Diabetes Care. 2007 Feb;30 (2):280-5
– Bodles AM, Banga A, Rasouli N, Ono F, Kern PA, Owens RJ. Pioglitazone increases secretion of high-molecular-weight adiponectin from adipocytes. Am J Physiol Endocrinol Metab. 2006 Nov;291 (5):E1100-5
– Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2003;278:45021-45026.
-Chen TH, Chen L, Hsieh MS, Chang CP, Chou DT, Tsai SH. Evidence for a protective role for adiponectin in osteoarthritis. Biochim Biophys Acta. 2006 Aug;1762 (8):711-8
– Ebinuma H, Miyazaki O, Yago H, Hara K, Yamauchi T, Kadowaki T. A novel ELISA system for selective measurement of human adiponectin multimers by using proteases. Clin Chim Acta. 2006 Oct;372 (1-2):47-53
– Fisher FM, Trujillo ME, Hanif W, Barnett AH, McTernan PG, Scherer PE, Kumar S. Serum high molecular weight complex of adiponectin correlates better with glucose tolerance than total serum adiponectin in Indo-Asian males. Diabetologia. 2005 Jun;48 (6):1084-7
– Glintborg D, Frystyk J, Hojlund K, Andersen KK, Henriksen JE, Hermann AP, Hagen C, Flyvbjerg A, Andersen M. Total and high molecular weight (HMW) adiponectin levels and measures of glucose and lipid metabolism following pioglitazone treatment in a randomized placebo-controlled study in polycystic ovary syndrome. Clin Endocrinol (Oxf). 2008 Feb;68 (2):165-74
– Haqq AM, Muehlbauer M, Svetkey LP, Newgard CB, Purnell JQ, Grambow SC, Freemark MS. Altered distribution of adiponectin isoforms in children with Prader-Willi syndrome (PWS): association with insulin sensitivity and circulating satiety peptide hormones. Clin Endocrinol (Oxf). 2007 Dec;67 (6):944-51
– Hara K, Horikoshi M, Yamauchi T, Yago H, Miyazaki O, Ebinuma H, Imai Y, Nagai R, Kadowaki T. Measurement of the high-molecular weight form of adiponectin in plasma is useful for the prediction of insulin resistance and metabolic syndrome. Diabetes Care. 2006 Jun;29 (6):1357-62
– Hattori Y, Hattori S, Akimoto K, Nishikimi T, Suzuki K, Matsuoka H, Kasai K. Globular adiponectin activates nuclear factor-kappaB and activating protein-1 and enhances angiotensin II-induced proliferation in cardiac fibroblasts. Diabetes. 2007 Mar;56 (3):804-8
– Hug C, Lodish HF. The role of the adipocyte hormone adiponectin in cardiovascular disease. Curr Opin Pharmacol. 2005 Apr;5 (2):129-34
– Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, Lodish HF. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci U S A. 2004 Jul 13;101 (28):10308-13
– Hui CK, Zhang HY, Lee NP, Chan W, Yueng YH, Leung KW, Lu L, Leung N, Lo CM, Fan ST, Luk JM, Xu A, Lam KS, Kwong YL, Lau GK. Serum adiponectin is increased in advancing liver fibrosis and declines with reduction in fibrosis in chronic hepatitis B. J Hepatol. 2007 Aug;47 (2):191-202
– Iwashima Y, Horio T, Kumada M, Suzuki Y, Kihara S, Rakugi H, Kawano Y, Funahashi T, Ogihara T. Adiponectin and renal function, and implication as a risk of cardiovascular disease. Am J Cardiol. 2006 Dec 15;98 (12):1603-8
– Kobayashi H, Ouchi N, Kihara S, Walsh K, Kumada M, Abe Y, et al. Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circulation research. 2004 Mar 5;94(4):e27-31.
– Kobayashi H, Ouchi N, Kihara S, Walsh K, Kumada M, Abe Y, Funahashi T, Matsuzawa Y. Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circ Res. 2004 Mar 5;94 (4):e27-31
– Kobayashi H, Ouchi N, Kihara S, Walsh K, Kumada M, Abe Y, Funahashi T, Matsuzawa Y, Ferre P. Selective Suppression of Endothelial Cell Apoptosis by the High Molecular Weight. – Komura N, Kihara S, Sonoda M, Kumada M, Fujita K, Hiuge A, Okada T, Nakagawa Y, Tamba S, Kuroda Y, Hayashi N, Sumitsuji S, Kawamoto T, Matsumoto S, Ouchi N, Arita Y, Okamoto Y, Shimomura I, Funahashi T, Matsuzawa Y. Clinical significance of high-molecular weight form of adiponectin in male patients with coronary artery disease. Circ J. 2008 Jan;72 (1):23-8
– Kusminski CM, McTernan PG, Schraw T, Kos K, O’Hare JP, Ahima R, Kumar S, Scherer PE. Adiponectin complexes in human cerebrospinal fluid: distinct complex distribution from serum. Diabetologia. 2007 Mar;50 (3):634-42
– Lara-Castro C, Luo N, Wallace P, Klein RL, Garvey WT. Adiponectin multimeric complexes and the metabolic syndrome trait cluster. Diabetes. 2006 Jan;55 (1):249-59
– Liu Y, Retnakaran R, Hanley A, Tungtrongchitr R, Shaw C, Sweeney G. Total and high molecular weight but not trimeric or hexameric forms of adiponectin correlate with markers of the metabolic syndrome and liver injury in Thai subjects. J Clin Endocrinol Metab. 2007 Nov;92 (11):4313-8
– Lo J, Bernstein LE, Canavan B, Torriani M, Jackson MB, Ahima RS, Grinspoon SK. Effects of TNF-alpha neutralization on adipocytokines and skeletal muscle adiposity in the metabolic syndrome. Am J Physiol Endocrinol Metab. 2007 Jul;293 (1):E102-9
– Matsuda M, Shimomura I, et al. Role of adiponectin in preventing vascular stenosis. The missing link of adipo-vascular axis. J Biol Chem. 2002;277:37487-37491.
– Modan-Moses D, Stein D, Pariente C, Yaroslavsky A, Ram A, Faigin M, Loewenthal R, Yissachar E, Hemi R, Kanety H. Modulation of adiponectin and leptin during refeeding of female anorexia nervosa patients. J Clin Endocrinol Metab. 2007 May;92 (5):1843-7
– Nakano Y, Tajima S, Yoshimi A, Akiyama H, Tsushima M, Tanioka T, Negoro T, Tomita M, Tobe T. A novel enzyme-linked immunosorbent assay specific for high-molecular-weight adiponectin. J Lipid Res. 2006 Jul;47 (7):1572-82
– Nakatani H, Hirose H, Yamamoto Y, Saito I, Itoh H. Significance of leptin and high-molecular weight adiponectin in the general population of Japanese male adolescents. Metabolism. 2008 Feb;57 (2):157-62
– Neumeier M, Sigruener A, Eggenhofer E, Weigert J, Weiss TS, Schaeffler A, Schlitt HJ, Aslanidis C, Piso P, Langmann T, Schmitz G, Scholmerich J, Buechler C. High molecular weight adiponectin reduces apolipoprotein B and E release in human hepatocytes. Biochem Biophys Res Commun. 2007 Jan 1

